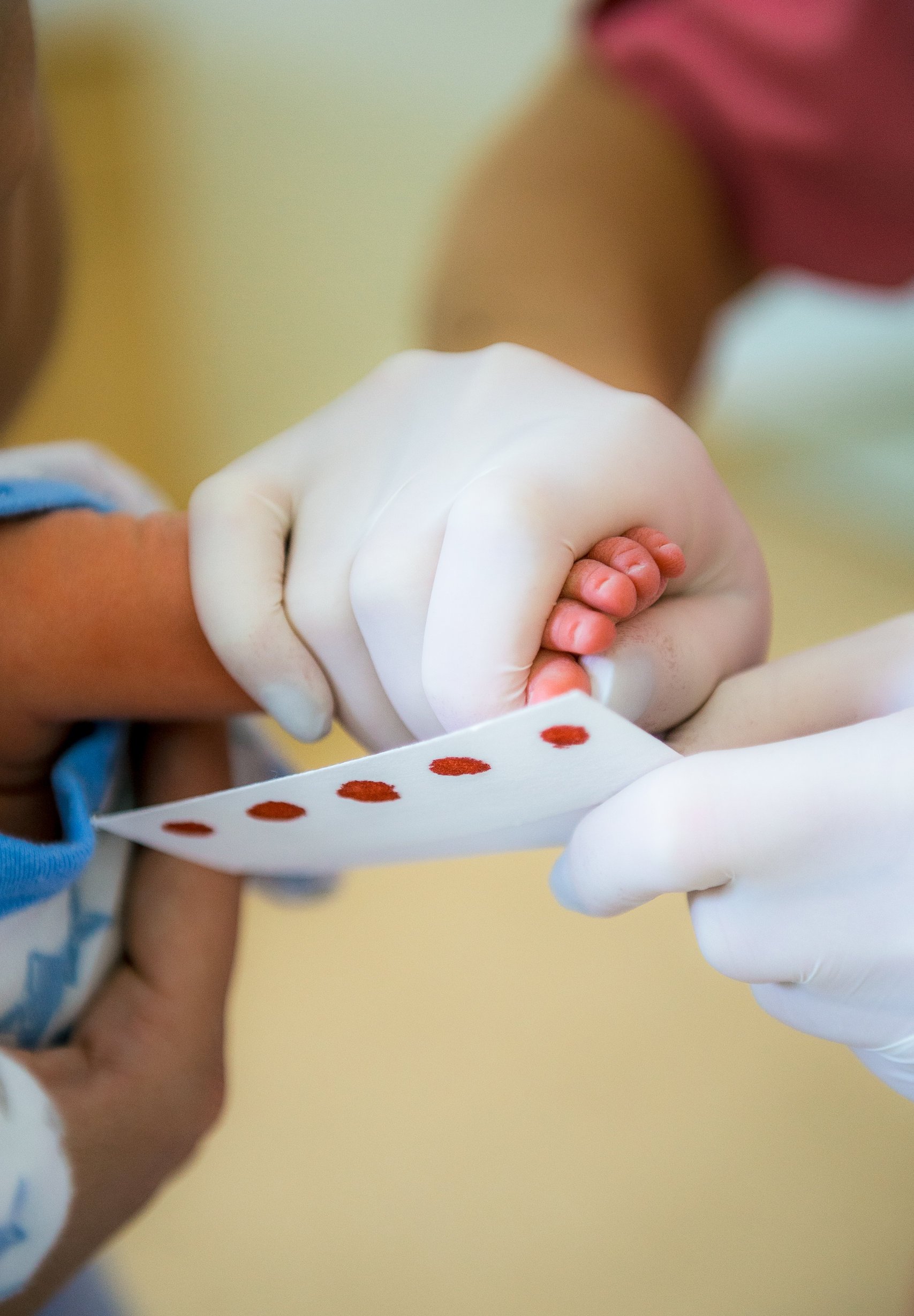
With the advent of low-cost methods to test for three common chromosome 15q disorders—Prader-Willi syndrome (PWS), Angelman syndrome, and Dup15q—researchers in several countries are now evaluating their use in newborn screening (NBS) panels. Various studies are currently underway that will provide data on the practicality, clinical utility and cost-effectiveness of this approach.
In the United States and Europe, as well as high-resource Asia Pacific countries like Australia and Singapore, virtually all newborns undergo NBS as part of state-sponsored programmes. The standard panel, which is conducted on dried blood spots collected within the newborn’s first week of life, typically includes more than 25 conditions, most of which are inborn errors of metabolism. In recent years, some countries have expanded their panels to include other rare diseases like cystic fibrosis, congenital heart disease, pompe disease and spinal muscular atrophy (SMA).
Much like the other conditions that are routinely covered in NBS panels, 15q disorders are life-limiting, potentially life-threatening, and sometimes hard to detect early through clinical examinations. While individually they may be rare, they are more common than some of the ultra-rare diseases already included in standard NBS panels.
A new method makes routine NBS for 15q disorders feasible
Until recently, 15q disorders were typically detected in the initial weeks or months of life based on clinical symptoms that include hypotonia (or weak and “floppy” musculature), feeding difficulties, and other developmental delays. Clinicians who notice these symptoms and understand their potential significance will typically order a DNA methylation test to determine their cause.
The problem with standard DNA methylation test methods for 15q disorders is that they can be expensive and require special expertise that may not be available outside of designated centres of excellence. They also do not reliably work on limited newborn blood spot material available, so they require additional blood draws. Finally, in the absence of routine screening, it is also possible that clinicians and parents may not notice the warning signs and thus lose opportunities for early intervention.
“Data from the PWS Global Registry suggests that roughly 20% of PWS cases in the United States are not detected early,” says Dr Theresa Strong, Director of Research at the Foundation for Prader-Willi Research (FPWR), a research and advocacy group.
A new, low-cost methylation test developed by Dr David Godler, a molecular geneticist who leads the Diagnosis and Development Laboratory at the Murdoch Children’s Research Institute (MCRI) in Australia, holds potential to address these challenges. Referred to as Methylation Specific Quantitative Melt Analysis (MS-QMA), it was initially applied to Fragile X syndrome, but modified in 2016 to cover the three main 15q disorders.
In January of this year, Dr Godler and colleagues published the results of a multi-year validation study in JAMA Network Open [1]. Using materials from 109 PWS, 48 Angelman Syndrome, and 9 Dup15q patient samples, the study demonstrated the feasibility of using the MS-QMA method and the confirmatory workflow for routine NBS of 15q disorders on approximately 17,000 infants from the general population. The study also indicated that the cost and performance of the test and the confirmatory workflow were roughly in line with other conditions included in NBS programmes.
Now, in a new study led by RTI International in the United States, the MS-QMA approach for 15q disease detection is being evaluated through Early Check [2], a programme that provides free NBS services in order to gather evidence about new tests. Located in the state of North Carolina, the programme previously ran evaluations on Fragile X and SMA testing, and is currently investigating muscular dystrophy testing.
Planning for population scale studies is also underway in Australia to examine clinical utility and economic benefits of MS-QMA and the confirmatory genomic workflow as part of an extended newborn screening approach, according to Dr Godler.
What are the opportunities and challenges?
By identifying the presence of disorders before signs and symptoms become apparent, NBS programmes enable early intervention that can save lives and improve outcomes dramatically. Yet due to the costs and challenges of implementing NBS tests at scale, as well as the availability of potential alternative approaches to screening, relatively few conditions ultimately make it onto state-sponsored panels.
In a recent summit on NBS at the International Prader Willi Syndrome Organisation (IPWSO), Dr Daniel Driscoll, a Professor in the Department of Paediatric Genetics and Metabolism at the University of Florida, said that new tests are only added onto state-sponsored panels if there is a compelling reason to do so [3]. For PWS specifically, he noted that the presence of obvious clinical signs in many patients may obviate the need for routine NBS, and that even if a case can be made for the other 15q diseases, it could take years for guidelines to change.
At the same summit, Dr Godler pointed out that many cases of PWS are still missed, especially in low-resource settings where health workers may not know about 15q diseases or have the tools to test for them [4]. Since early intervention with growth hormone treatment and various behavioural therapies can make a big difference in PWS outcomes, late or missed diagnosis can lead to suboptimal and sometimes tragic results.
These missed cases may lead to skewed population prevalence estimates for 15q diseases, leading to a possible underestimation of the problem and misallocation of resources from healthcare systems. “Prevalence estimates are all over the place,” says Dr Godler. “With maternal age changing in many countries, we may also be seeing changes in prevalence that could impact the numbers.”
“We are also finding that the proportion of PWS affected children due to uniparental disomy [a subtype of PWS] is now about half as compared to 10 years ago when this was about 30%,” added Dr Godler. “This means that alternative genomic testing like NIPT is largely restricted to identifying deletions that cause PWS and AS, and will miss a significant number of affected individuals who would be identified through methylation testing.”
Several participants at the summit expressed concern about false positives and the potential unnecessary suffering that could cause to parents. Indeed, positive predictive value (PPV) for the first-tier MS-QMA test was measured at 67% for Angelman, 33% for PWS, and 44% for combined detection of chromosome 15 imprinting disorders.
However, Dr Godler emphasised that absolute PPV is actually close to 100% if you take into account the entire workflow, including confirmatory genomic and methylation testing that can be conducted on a second punch from the same blood spot for a small number of samples shortlisted by MS-QMA, and before any results are returned to parents.
Even so, Dr Godler acknowledges that more evidence is needed to truly evaluate the cost-benefit of routine NBS for 15q disorders. Yet given the looming prospect of late or missed diagnosis, and with new medicines in development to treat 15q disorders, routine NBS could be a game changer to improve outcomes for the individuals that suffer from these conditions, as well as for their families and for health systems.
This article is the first in a series on newborn screening and rare disease diagnostics. For more information about this series, please contact Will Greene, Healthcare Engagement Manager (Asia Pacific) at [email protected].
References:
[1] Godler DE, Ling L, Gamage D, et al. Feasibility of Screening for Chromosome 15 Imprinting Disorders in 16 579 Newborns by Using a Novel Genomic Workflow. JAMA Netw Open. 2022;5(1):e2141911. doi:10.1001/jamanetworkopen.2021.41911
[2] FPWR, FAST, ASF, and Duq15q Unite to Fund Newborn Screening Grant, Foundation for Prader-Willi Research Available at: <https://www.fpwr.org/blog/fast-asf-dup15q-and-fpwr-unite-to-fund-newborn-screening-grant>
[3] Overview of NBS and a Brief Discussion of PWS by Professor Daniel Driscoll at the Summit on Newborn screening for Prader-Willi syndrome and other chromosome 15 abnormalities, International Prader-Willi Syndrome Organisation. Available at: <https://www.youtube.com/watch?v=dlKNKJX8BGQ&t=4s>
[4] Newborn Screening for Prader-Willi syndrome by Associate Professor David Godler at the Summit on Newborn screening for Prader-Willi syndrome and other chromosome 15 abnormalities, International Prader-Willi Syndrome Organisation. Available at: <https://www.youtube.com/watch?v=9esfE26uLrI>