Sepsis is one of the top reasons for admission to intensive care units (ICUs). This life–threatening, systemic reaction to infection is also a major cause of morbidity and mortality in hospitalised patients. Proper diagnosis of the cause of sepsis is crucial for making a decision on an appropriate treatment, and can increase the odds of patient survival. In the first 12 hours of sepsis care, each 5-minute delay in proper therapy increases the mortality rate by ~1% [1].
Physicians who treat sepsis patients need to know if the high fever is caused by infection, systemic inflammatory response syndrome (SIRS), or other causes. In the case of infection, it should be determined whether the illness is caused by a viral, fungal or bacterial infection. Once therapy has begun, physicians must be able to keep track of a patient’s antibiotic response and decide when it is safe to stop therapy. This is where biomarkers can play a key role in sepsis management.
Biomarkers—biological indications of the state of a patient’s health—must be highly sensitive and highly specific for a condition as severe as sepsis. They must fluctuate according to the patient’s response to treatment (even before symptoms change), and be both easy to use and affordable.
Procalcitonin as a sepsis biomarker
Although more than 200 biomarkers have been studied for sepsis, there are only three major proteins commonly used for sepsis diagnosis and prognosis: interleukin 6 (IL-6), C–reactive protein (CRP) and procalcitonin (PCT). These three biomarkers have different onsets, peaks and half-lives.
PCT has an onset at 2-6 hours, a peak at about 24 hours, and a half-life of 24 hours [2]. These characteristics make PCT one of the most suitable biomarker proteins for practical use [3].
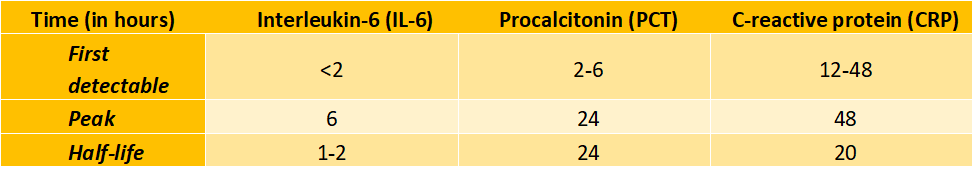
Bacterial toxins (both gram positive and negative) and cytokines stimulate PCT production in the body, while interferon–α (present during viral infections) inhibits PCT secretion, making it possible to use PCT to distinguish between viral and bacterial infections. Studies show that PCT has 89% sensitivity, 94% specificity, 90% negative predictive value (NPV), and 94% positive predictive value (PPV) for bacterial sepsis – better than IL-6, CRP and lactate biomarkers [7].
PCT in sepsis diagnosis and prognosis
PCT has been well studied in sepsis diagnosis. It is known to improve the accuracy of early clinical sepsis diagnosis compared to conventional clinical assessment. It also differentiates viral from bacterial community-acquired pneumonia [8], as well as invasive fungal infection from bacterial infection [9].
Beyond helping with diagnosis, rising PCT levels correlate with the severity of sepsis [10]. Thus, PCT can be used for severity stratification in patients with suspected sepsis, severe sepsis and septic shock [11]. PCT levels can be also useful for the management of patients after surgery or transplant, and in cases of peritonitis [12].
In addition, a 30% decrease in PCT levels between Day 2 and Day 3 appears to be a good prognostic indicator of effective antibiotic therapy and is associated with better survival. PCT-guided algorithms for antibiotic usage, when combined with clinical assessment, have been shown to reduce the duration of antibiotic therapy and ICU stays, without negatively impacting mortality [13].
Limitations of PCT for use in sepsis management
Despite the major advantages of PCT in sepsis diagnosis and prognosis, it has limitations. False positives are possible in several patient groups:
- Newborns less than 48 hours old
- Patients with primary inflammation syndrome from severe trauma, burns or major surgery
- Cancer patients, particularly those with medullary C-cell cancers of the thyroid, small-cell lung carcinomas, bronchial carcinomas and advanced liver cancer
- Prolonged shock patients
- Patients who have received treatments (such as anti-lymphocyte globulins) that can cause a “cytokine storm”
There also needs to be more research to refine cut-off points for differences in PCT among patient populations, by patient setting, in the very elderly, and with different assay products.
Incorporating PCT into sepsis management
Many of the clinical practice guidelines used around the world today include PCT. For example, the 2012 and 2016 Surviving Sepsis Campaign guidelines [14] recommend that PCT be used to guide duration of antimicrobial treatment in sepsis patients, and the discontinuation of empiric antibiotics in patients who, with time, appear to have little evidence of sepsis.
Although PCT cannot be the exclusive factor considered during sepsis diagnosis and treatment decisions, it can be a helpful, practical tool for improving patient management and outcomes.
[1] Funk, D. J., Kumar, A. (2011). Antimicrobial therapy for life-threatening infections: speed is life. Critical care clinics, 27(1), pp.53–76.
[2] Meisner, M. (1996). PCT, procalcitonin: a new, innovative infection parameter : biochemical and clinical aspects. Berlin, Brahms Diagnostica.
[3] Reinhart, K., Meisner, M. (2011). Biomarkers in the critically ill patient: procalcitonin. Critical care clinics, 27(2), pp.253–263.
[4] Meisner M. (2002). Pathobiochemistry and clinical use of procalcitonin. Clinica chimica acta; international journal of clinical chemistry, 323(1-2), pp.17–29.
[5] Morgenthaler, N. G., et al., (2002). Detection of procalcitonin (PCT) in healthy controls and patients with local infection by a sensitive ILMA. Clinical laboratory, 48(5-6), pp.263–270.
[6] Müller, B., et al., (2001). Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. The Journal of clinical endocrinology and metabolism, 86(1), pp.396–404.
[7] Simon, L., et al., (2004). Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 39(2), pp.206–217.
[8] Cuquemelle, E., et al., (2011). Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive care medicine, 37(5), pp.796–800.
[9] Aznar-Oroval, E., et al., (2010). Diagnostic value of procalcitonin, interleukin 8, interleukin 6, and C-reactive protein for detecting bacteremia and fungemia in cancer patients. Enfermedades infecciosas y microbiologia clinica, 28(5), pp.273–277.
[10] Harbarth, S., et al., (2001). Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. American journal of respiratory and critical care medicine, 164(3), pp.396–402.
[11] Krüger, S., et al., (2008). Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. The European respiratory journal, 31(2), pp.349–355.
[12] Meisner, M., et al., (1999). Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Critical care (London, England), 3(1), pp.45–50.
[13] Charles, P. E., et al., (2009). Procalcitonin kinetics within the first days of sepsis: relationship with the appropriateness of antibiotic therapy and the outcome. Critical care (London, England), 13(2), R38.
[14] Rhodes, A., et al., (2017). Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med, 43, pp.304–377.
This article is based on a presentation “Get Ahead of Sepsis” at Roche Scientific Days 2018: Empowering Lab Leadership to the Next Level in Dusit Thani Hua Hin, Thailand.