Roche Diagnostics Asia Pacific is pleased to announce the release of the latest results for its Asia Pacific (APAC) Laboratory Benchmarking Survey, the largest and most impactful study on laboratory operations in the region. Results are now available on Lab Insights, a digital platform that provides data and content for the clinical lab community.
Initially launched in 2010, the Asia Pacific Laboratory Benchmarking Survey allows labs to assess their performance on a wide range of quality, speed and cost indicators, helping them to identify areas for improvement and maintain competitive advantage. More than 3,500 labs from over 20 countries have participated in the survey since inception, providing a rich trove of longitudinal industry data.
The latest results, which cover all the data collected in 2019, include more than 1,150 unique responses—our largest annual sample yet. By visiting Lab Insights, clinical labs can take the survey and view results via interactive dashboards that enable comparisons against different peer groups. Some key takeaways can be seen in the infographic below.
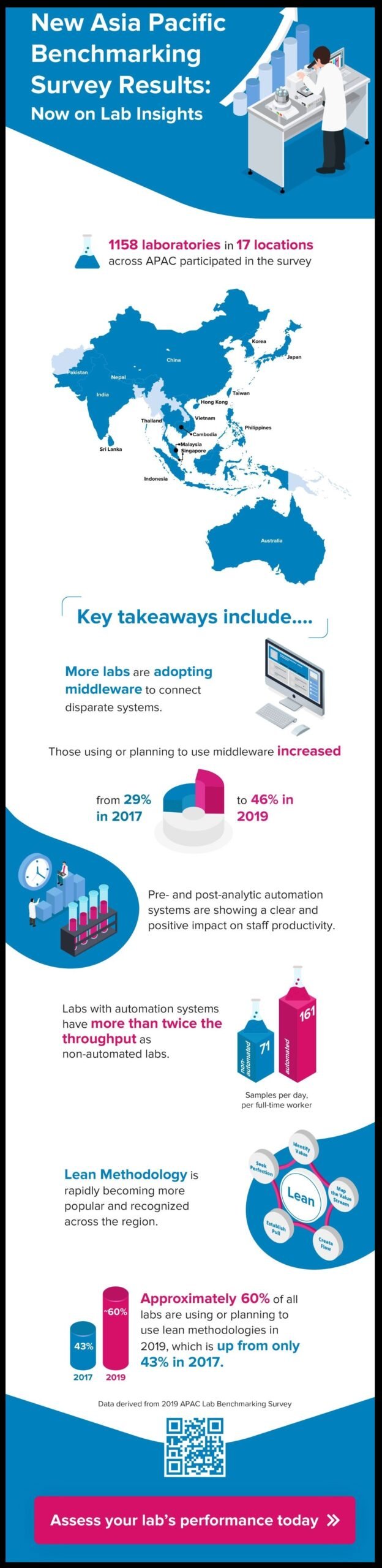
As in previous years, the current survey covers key metrics related to quality assurance, accreditation, continuous improvement, IT infrastructure, turnaround time, laboratory setup and productivity. Starting in 2019, it also introduced several new topics, including processes and KPIs for sample handling, sample rejection, serum quality monitoring, clinical decision support and critical results reporting.
Since 2017, the survey inspired three peer-reviewed publications that highlighted key trends unfolding across the region. This year, Roche Diagnostics will release a series of new reports based on the survey data, including coverage of regional dynamics, as well as deep dives on specific markets like China, Korea, Thailand and India.
For more analysis, visit Lab Insights, to take the survey, view your results and see the latest content. Stay tuned also for the release of new surveys that are specifically geared for labs operating in the Molecular Diagnostics and Anatomic Pathology segments, as well as our forthcoming Clinical Lab Transformation Survey, which helps laboratory and hospital CEOs to understand the key strategies that will impact the future relevance of the lab.
-
-
- For more information, please contact Wesley Wong, Lab Insights Consulting Manager, Roche Diagnostics Asia Pacific ([email protected])
-








