In 2016, laboratory leaders from five major healthcare systems in the United States founded the Project Santa Fe Foundation (PSFF), a non-profit organisation with a mission to chart a path for the future of the clinical laboratory. Their vision is a world in which labs deliver massive value for healthcare systems everywhere by leveraging their unique data and capabilities. Their efforts, which they collectively refer to as the Clinical Lab 2.0 Initiative [1], aim to make this vision a global reality by improving clinical outcomes of populations, managing population risk and reducing the overall cost of delivering healthcare.
In just three years, PSFF built a Board of Directors [1] that includes representatives from many of America’s leading healthcare systems* and brought together a global community of like-minded lab professionals that share their ambitious vision. They launched pilot projects, published papers in peer-reviewed journals, and ran workshops that highlight the various ways that labs can play a bigger role in healthcare systems everywhere. They also initiated dialogue with leading payers, providers and other stakeholders to showcase the possibilities and forge the multi-stakeholder collaborations that will enable lab-driven progress.
The community includes participants from around the world, but most of their key efforts so far have been focused on the United States. Yet the organisation sees major opportunities in Asia and other continents. “Lab 2.0 has no borders,” according to Khosrow Shotorbani, President and Executive Director of PSFF and a leading voice in the Clinical Lab 2.0 community.
Building a movement
Shotorbani, along with initially four other members, co-founded PSFF while he was CEO at Tricore Reference Laboratories, the largest medical laboratory in New Mexico. With over thirty years of experience in the laboratory industry, he knew the current business model is not sustainable and that labs could do much more than simply process samples and return results. Even so, many lab professionals still clung to traditional “transactional” business models that undervalue and commoditise laboratory services and expertise.
“The main problem with clinical labs is that we don’t have a seat at the table,” observes Shotorbani. “We like to sit in the basement of the health system rather than at the table in senior management meetings. That’s a myopic view, and it has to change.”
PSFF determined that for labs to get a seat at the table, they need to build on Clinical Lab 1.0—the traditional transaction-based model that most labs still use today—to Clinical Lab 2.0, which leverages patient-centric longitudinal clinical laboratory data as a catalyst to manage population health in a value-based healthcare environment. Potential uses of that data include:
- Stratification of risk by population against prevalence of conditions
- Identification and closure of care gaps
- Early identification of high-risk patients
- Facilitating targeted intervention before the patient is admitted in the emergency department or hospital
Shotorbani felt that industry leaders needed to collaborate more to realise this potential. As the founding President and Executive Director of PSFF, one of his first initiatives was to co-author a white paper [2] in Academic Pathology, an open-access journal sponsored by the Association of Pathology Chairs, that described the many ways that labs can take on a more “integrative” model and work to improve population health, support preventative medicine and reduce the cost of care.
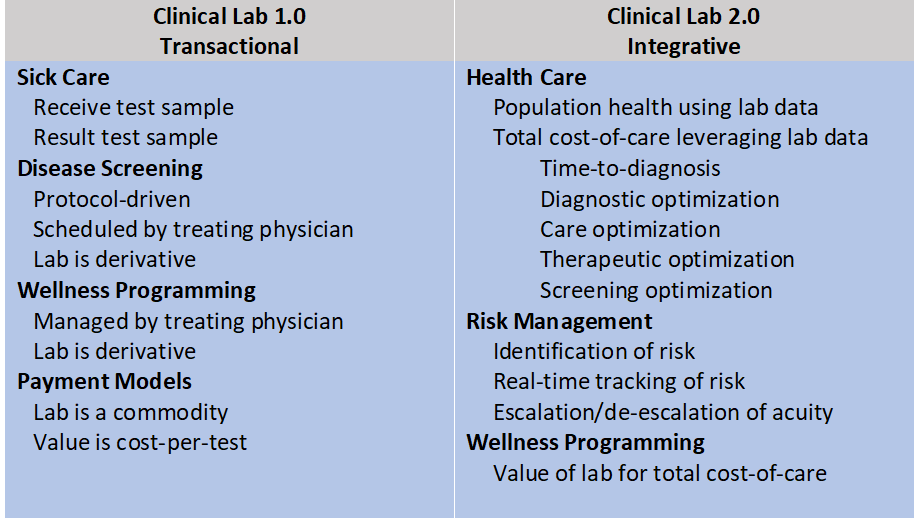
“Lab 1.0 is about accuracy, turn-around time, cost per unit and producing results for the physician,” says Shotorbani. “Clinical labs have significant opportunities to help solve healthcare challenges through Lab 2.0 which is about post-diagnostic computation and analysis—turning data into clinical action. It’s about connecting the dots over time to produce meaningful insight to improve outcomes while minimising financial risk and thus achieve alignment with the strategic goals of healthcare systems.”
Beyond publications, thought leadership, and changing conversations, PSFF also set out to conduct “multi institutional demonstration projects” that would build the evidence base for this new paradigm of care, intervention, prevention and cost avoidance. This included case studies on using laboratory data to help predict sepsis, develop early interventions for sepsis prevention, help manage the American opioid epidemic and improve prenatal care. These case studies and several others were presented at PSFF’s annual Clinical Lab 2.0 workshop in 2018. The next workshop will be held in Chicago, IL on 3-5 November 2019 (registration is available at this link).
Bringing Lab 2.0 to Asia
After building significant traction in the United States, Shotorbani increasingly has his sights set on other regions, believing lab 2.0 has no borders. He is particularly excited about the opportunities for labs in Asia.
“I’ve shared the Clinical Lab 2.0 vision with its unique value proposition and business model around the world, including four presentations in South Asia in the last 18 months, and I can say that traction in the Clinical Lab 2.0 conversation is moving faster in Asia than in the United States,” notes Shotorbani. “This is in part because many Asian countries already have single-payer systems with a high interest in managing costs and outcomes.”
To help spread the message, Shotorbani plans to present at various events later this year. These lectures will provide the broad vision of the Clinical Lab 2.0 movement and activities of PSFF, as well as plans to deliver an in-depth case study that provides an actionable roadmap for how to implement a Clinical Lab 2.0 business model and strategic plan.
“We laboratorians get very focused on standard operating procedures at the operational level for very critical reasons to assure accuracy of results, but we don’t yet have a well-established playbook for determining our value and impact at the institution and healthcare systems level,” says Shotorbani. “This is not just in the US—we must get out of the health system’s basement and share our measurable value aligned with drivers at the enterprise organisation, globally.”
The three key pillars of the Clinical Lab 2.0 initiative provide a foundation for that recipe and include:
- CL2.0 Leadership: defining leadership skills and the knowledgebase needed to meet Clinical Lab 2.0 objectives
- CL2.0 Standards: measuring what matters in a Clinical Lab 2.0 operation and developing a new set of KPIs that are meaningful outside of the lab
- CL2.0 Evidence: demonstrating both the economic and clinical value of Clinical Lab 2.0 in multi-institution, collaborative projects
“Our work at PSFF is to develop a playbook and KPIs that help us to plan our future as an industry and track our progress in getting there with the result of improving population health, reducing the cost of healthcare and establishing the valuation of clinical laboratory services in the next era of global healthcare.”
*Members of the PSFF board include Geisinger Health System, Henry Ford Health System, Intermountain Healthcare Central Laboratory, The Mayo Clinic, NorthShore University HealthSystem, Northwell Health System, Seattle Children’s Hospital, TriCore Reference Laboratories, University of Vermont Medical Center and Lab 2.0 Strategic Services, LLC.
[1] Clinical Lab 2.0, A Project Santa Fe Foundation Initiative
[2] Crawford. M.J., et al. 2017. Improving American Healthcare Through “Clinical Lab 2.0”: A Project Santa Fe Report. Academic Pathology. 4, pp.1-8.







