In June 2020, author and futurist Tony Estrella published A Vision for APAC in 2050, a whitepaper to stimulate conversation on the future of health in this region. The whitepaper originally appeared on FutureProofing Healthcare, a platform for sharing data and insights to drive evidence-based decision making. This article extends the concepts from this whitepaper to explore how the function of clinical labs may evolve in the year 2050.
Subscribe to Lab Insights’s latest news and update
Join over 3,000 HCPs receiving insights in their inbox every week.
Vision 2050: real-time health data solves a global health crisis
Olivia and Kalpana held their hands high above their heads as thunderous applause rose from the World Health Summit 2050 audience. With the completion of their joint keynote presentation, 15 challenging but fruitful years of a deep collaboration between their countries came to a satisfying conclusion. The words hovering in the holographic banner floating above their heads summed up why they both beamed in delight: “The Ministries of Preventative Health from India and Singapore announce an innovative programme to eliminate cancer.”

A few minutes later, they sat together backstage, catching their breaths from all the excitement. Kalpana looked at her congratulatory messages and passed her phone to Olivia.
“You know who we have to call first, don’t you?”
“Of course, lah!” Olivia immediately tapped her screen to reach the global headquarters for the Digital Twin Bureau. “Without their data and real-time insights, none of this would have been possible!”
Recapping the journey from early clinical labs to Lab 3.0 in 2050
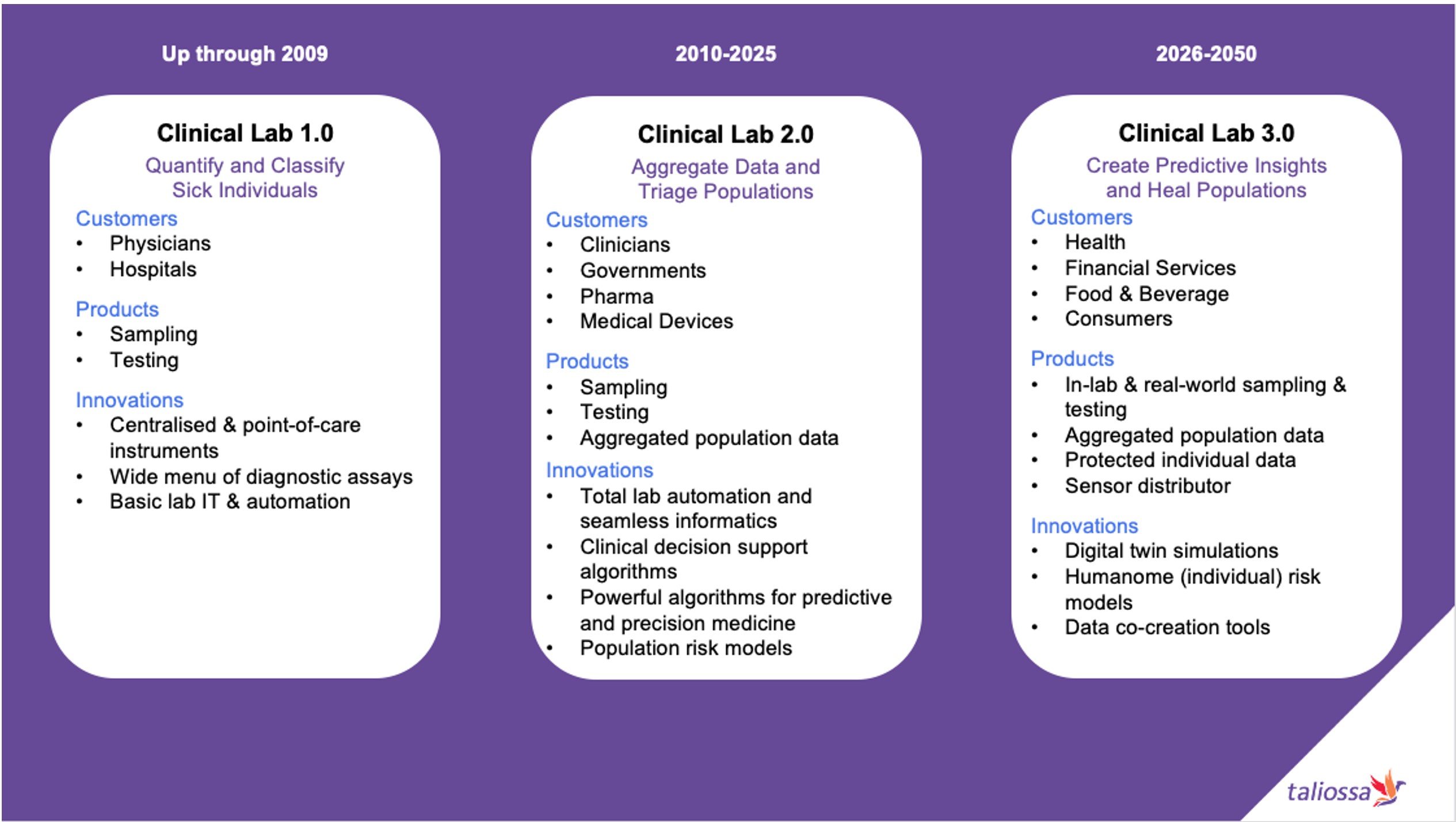
The history of taking observations of a person’s clinical symptoms goes as far back as ancient Egypt and Mesopotamia. As scientific knowledge increased, so did the sophistication of tests. The 19th century became a critical inflexion point for establishing Lab 1.0. For the next 150 years, the development of core equipment, such as the microscope and an ever-growing array of diagnostic instruments and assays, paved the way for clinicians to quantify and classify individuals objectively. A steady stream of innovations followed in sampling, testing, and human-led automation to help establish a base of clinical laboratory businesses.
Around 2010, a shift to Lab 2.0 started. After the COVID-19 pandemic began in 2020, the transition accelerated. This period of high demand for clinical labs exposed cracks in the global health infrastructure. As a result, two significant trends developed. First, the infusion of more technology—including robotics, artificial intelligence, and networking—created higher levels of machine-led automation within laboratories. The second shift came from the value of data as a business asset. This new clinical resource enabled greater co-creation and collaboration between clinical laboratories with other healthcare stakeholders such as hospitals, governments, pharma, and medical device companies.
By 2025, the Lab 2.0 model had moved well beyond the Lab 1.0 model of receiving test orders and optimising fulfilment. It established laboratories as a trusted data aggregator and operational partner. Case studies for the Lab 2.0 model included influencing key population health activities such as risk stratification, early disease identification, and systemic care gaps in health systems.
Over the next 25 years, the emergence of two new transformative changes caused the industry to redefine itself once again. Spurred by the explosion of consumer sensors with lab-on-a-chip capabilities, the data assets aggregated and analysed by clinical laboratories grew exponentially. The concept of a “laboratory” changed. Merging clinical knowledge with artificial intelligence and managing data farms led to insights becoming the new currency of operation.
As data grew, Lab 3.0 companies received interest from a more diverse set of customers for their insight-driven analyses and recommendations. Clinicians, hospitals, and governments remained as core buyers of these products. But the mountains of real-time data flowing into the virtual warehouses managed by Lab 3.0 companies had other benefits beyond testing and defining the right care interventions.
Groups sought out Lab 3.0’s innovation of “digital twin” simulations to model how to cure disease, improve health, and even explore safe genetic enhancements to help humans withstand the stresses of space travel. Other use-cases developed, adding new business models directly from consumers, including cross-licensing fees from Lab 3.0 companies who arbitered the use of an individual’s Humanome for improving their individual health outcomes as well as the overall Longevity Health of populations.
The following chart summarises the evolution of the journey to Lab 3.0.
The need for a neutral arbiter: the Digital Twin Bureau
In the early days of Lab 3.0, an industry challenge arose for how to create standards and resolve collaboration questions as the industry evolved its business model and suite of products and services. To guide the shift across individual clinical labs, business leaders proactively established a new type of entity within healthcare – the Digital Twin Bureau (DTB). Part standards body, part governance, and part data broker, the DTB helped provide structure and active management solutions.
As a standards and governance body, the organisation led the way in addressing critical questions in the following categories:

For example, over the 15 years where India and Singapore’s Ministries of Health collaborated, DTB helped bring together various stakeholders and even acted as a neutral data broker in three areas:
(1) Finding unique data:
- Identified trusted companies with implantable consumer products
- Provided lab-on-a-chip measurements for real-time glucose, hormone levels, individualised food toxicity based on gut biome, growth of beta amyloid plaques, and cardiovascular disease across a spectrum of people in both India and Singapore
(2) Running digital twin simulations:
- Identified trusted companies with scalable infrastructure and data privacy to run these complex models
- Established the standards for reporting on real-time data inputs which modelled preventative measures for lifestyle risk factors
- Benchmarked the outputs from work completed in Singapore and India for eliminating carcinogenic food and environmental causes with baseline data from other countries
- Worked with stakeholders to capture best practices for how to make genetic pre-dispositions actionable
(3) Evangelising thought leadership on individualised and population-level targeting measures to stop cancer:
- Gathered key leaders for knowledge sharing
- Supported consumer-facing organisations to inform them on new programmes to identify risk levels of individuals and support their approach for personalised interventions
Conclusion: What it took to achieve the vision of Lab 3.0
Change is not easy. This statement is especially true within health where there are many competing priorities and regulatory challenges which complicate growth.
From their earliest days through Lab 3.0, clinical labs have always dealt with data as a component of its products and services. Lab 3.0 is a natural landing point for how these organisations realised how to create both individual and population-level value to transform healthcare. Using this lens, achieving Lab 3.0 does not seem so disruptive, but rather a natural evolution of the industry.
The critical decisions that allowed individual businesses and stakeholders to follow this path for disruptive innovation and to achieve sustainable growth included:
- Shifting from a volume of tests completed and number of patients touched to creating impactful health outcomes across targeted populations
- Becoming consumer-centric through the use of technology
- Adapting to new business models with an expanded set of customers
- Integrating cultural context in the interpretation of data to make the resulting actions effective
These decisions ultimately led to the formation of an ecosystem including Lab 3.0 companies and the DTB, enabling people like Olivia and Kalpana to rid the world of cancer.
About FutureProofing Healthcare
FutureProofing Healthcare started in 2018 as a way to benchmark how health systems are performing today to prepare for the future. The programme is establishing a community for sharing insights and data so decisions can be driven by evidence, not emotion. COVID-19 is clearly putting a spotlight on this approach all over the world. The initiative is supported by Roche. Please visit www.futureproofinghealthcare.com to learn more.
About Tony Estrella
Tony Estrella is an author & futurist, business builder, and strategist in HealthTech. He is a Managing Director at Taliossa, enabling startups and publicly listed health companies to find product-market fit across Asia Pacific. Through advisory projects, supporting venture investors, and as an independent board director, Tony accelerates disruptive innovation in population health, cancer, brain health, and sleep science. He is featured by numerous media including the BBC World Service, Inside Asia, Digital Health Today, and The Healthcare Blog.
As an author, Tony predicts the future of healthcare as influenced by technologies including AI, smart devices, and robotics. His debut fiction novel Comatose explores the science of the mind and dreams in a globe-trotting speculative fiction thriller. He is currently working on its sequel. Please visit www.tonyestrella.com to learn more.








