In order to maintain high quality standards, many clinical labs in the Asia Pacific region participate in international accreditation and external quality control (EQC) programs. Participation is likely to increase in coming years, according to the latest results from the Asia Pacific Laboratory Benchmarking Survey, an annual survey by Roche Diagnostics that measures the operational effectiveness of clinical labs across the region.
Subscribe to LabInsights’s latest news and update
Join over 3,000 HCPs receiving insights in their inbox every week.

Thank you.
You have successfully subscribes to the newsletter!
Didn’t receive the email? Be sure to check your spam folder too!
You can contact [email protected] for further assistance
Alternatively, you can browse more content from ThoughtLeadership
Accreditation
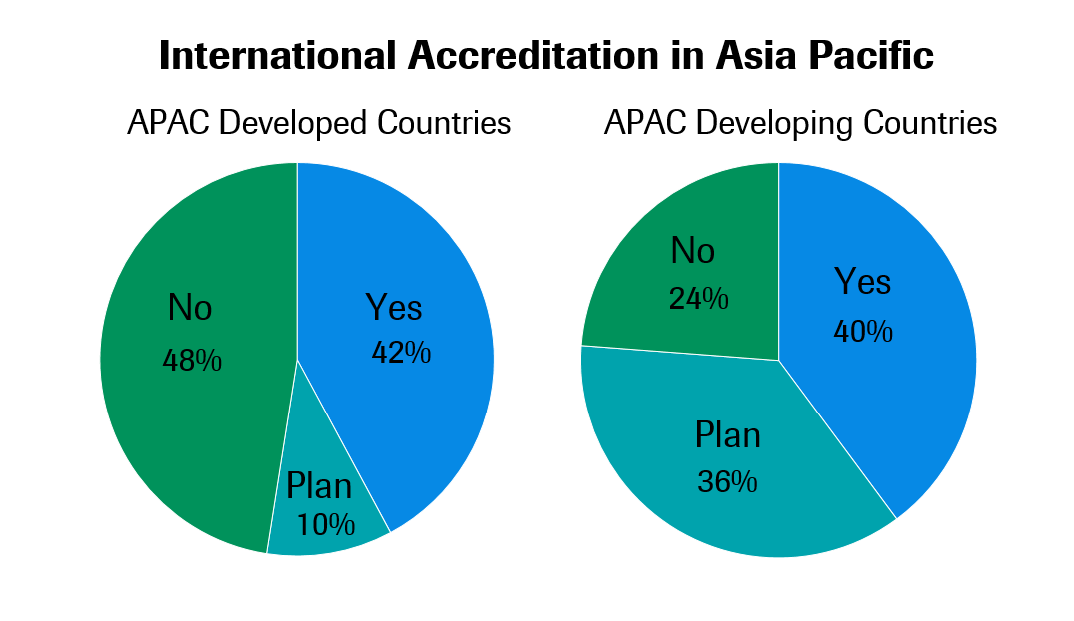
One way of ensuring laboratory quality is to adhere to standards set by internationally-respected accreditation bodies. In the past year’s survey, 40.3% of labs have earned some kind of international accreditation, with another 30.9% planning to pursue accreditation in the next 3 years.
Labs in developed markets currently have slightly higher rates of international accreditation than those in developing markets. This will likely change soon since more than a third of labs in developing markets plan to pursue international accreditation in the next three years, compared to only 10.3% in developed markets. International accreditation rates in Japan and Korea remain particularly low, even as other developed countries have relatively high rates of participation.
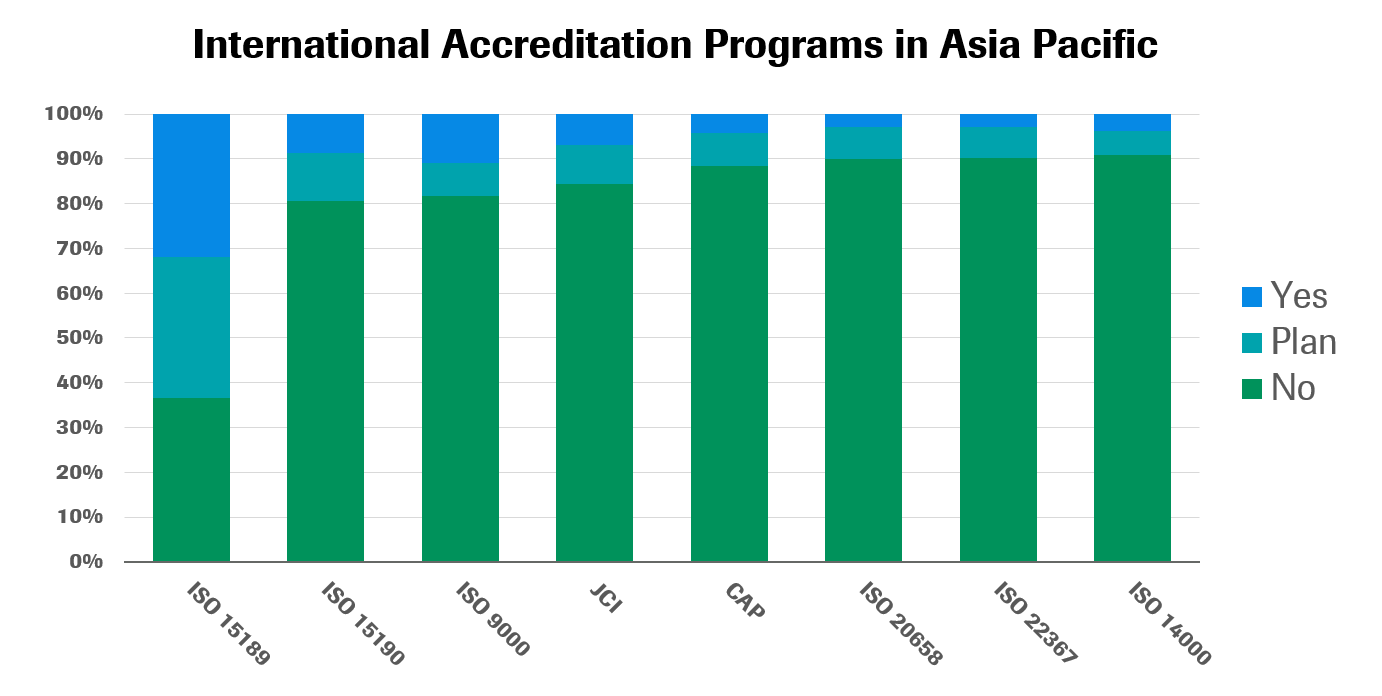
The most popular form of accreditation in Asia Pacific is ISO 15189, followed by ISO 9000 and ISO 15190. Other international accreditation standards have not yet gained significant traction in the region, though local standards are on the rise in some countries (see Thailand’s Medical Technology Standard Creates a Benchmark for Lab Quality in Lab Insights). These local standards may be particularly useful in countries where many labs do not have the time, expertise or resources to participate in more comprehensive international accreditation processes.
Within both developed and developing markets, private commercial labs tend to have higher levels of accreditation than public and private hospital labs, perhaps because independent labs serve a wider range of customers and thus face greater pressure to demonstrate quality. Large labs, which typically have greater resources for quality assurance, also tend to have higher levels of international accreditation than medium- and small-sized labs.
External Quality Control (EQC)
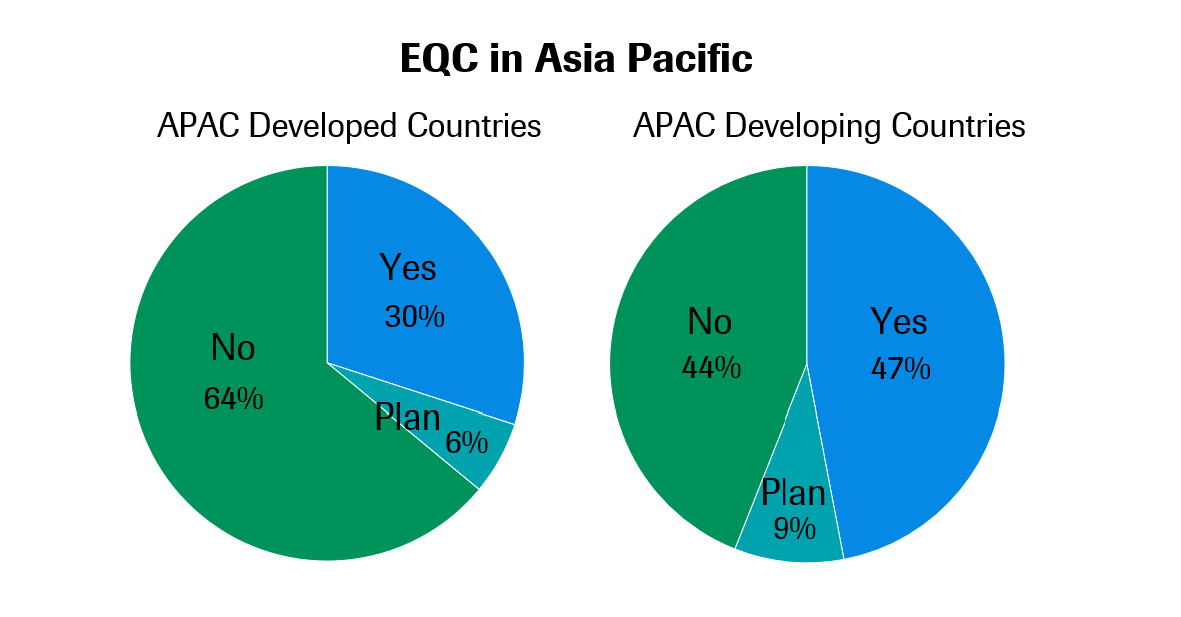
Alongside accreditation, international External Quality Control (EQC) programs are another common approach for maintaining operational effectiveness at clinical labs. 43.2% of all labs in the latest survey claimed to participate in an EQC program, with another 8.2% planning to implement them in the next three years.
International EQC programs are more popular in developing countries like Vietnam, Thailand and the Philippines. The only exception is China, where local EQC programs take precedence. Among developed countries, labs in Japan and Korea also have relatively low participation rates in international EQC programs.
The most popular EQC programs in Asia today are the External Quality Assurance Services from Bio-Rad (EQAS) and the Randox International Quality Assessment Scheme External Quality Assessment (RIQAS.EQA). Less popular are the EQA of the College of American Pathologists (CAP); the Medical Laboratory Evaluation of the American College of Pathologists (MLE); and the Quality Assurance Program of The Royal College of Pathologists (RCPA.QAP). CAP, for example, is only popular in a few key markets—including Hong Kong, Taiwan, Pakistan and Singapore.
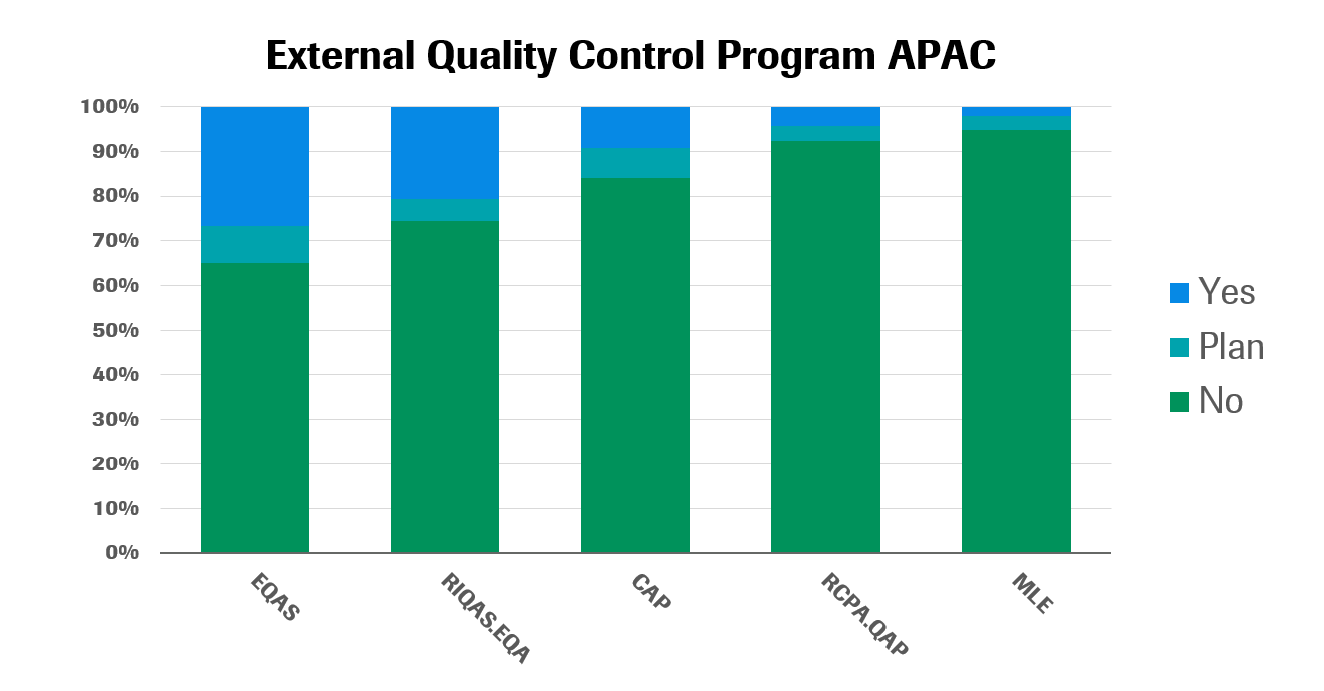
As with accreditation, commercial labs across most markets have a relatively high participation rates in international EQC programs. In developing countries, private hospital labs have higher participation rates than government hospitals, perhaps due to relatively low public sector resources. The opposite is true in developed countries, where government hospitals have higher participation than private hospitals.
In the coming weeks, Lab Insights will feature further analysis of key trends in Asia’s clinical laboratory space. To stay on top of these trends, please register for an account on Lab Insights and subscribe to our Roche Diagnostics Asia Pacific LinkedIn channel for updates. And if you have any specific questions about what these trends mean for your lab, contact Wesley Wong ([email protected]) for more information.