Laboratories everywhere seek to improve clinical outcomes while optimising cost and efficiency. To achieve this goal, they must align three objectives: high-quality testing, rapid performance and reasonable prices. In the real world, it’s easy to hit two of these targets but hard to attain all three. If something is done as quickly as possible and at a low price, you’re likely missing out on quality. If a task is done swiftly and with great care, it’s not going to be cheap.
So how can we deliver top-notch lab work at optimal speed while also keeping costs down? To meet this challenge, lab managers must take care to improve their internal processes while also remaining mindful of how their workflows fit with the changing needs of the broader health system.
Assess your internal and external costs
One important step to achieving excellence determining the cost of poor quality (CoPQ). This means evaluating how much is being spent on failure costs like missing specimens, retesting, technical repairs, recollected samples or incorrect analysis.
Jennifer Dawson, VP of Quality & Regulatory at Human Longevity Inc., champions using the COPQ calculator, an online device that helps track financial lab metrics. If only one particular issue is causing the bulk of the financial drains, it might be addressed easily, but if there are many concurrent issues, it may make sense to hire outside experts for more detailed operational reviews.
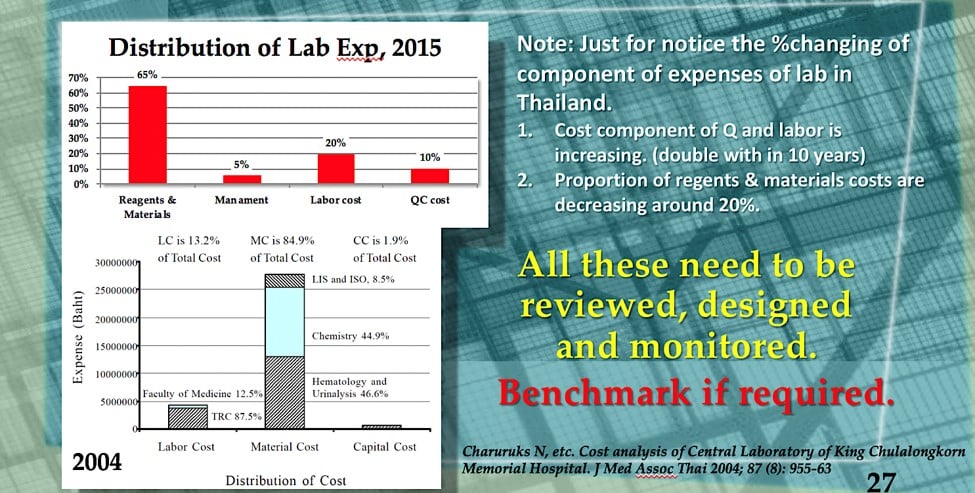
When calculating the CoPQ, Dawson recommends looking out for “soft internal failures”, such as low morale, excess downtime, and tests assigned to the wrong technician (see What’s Your Lab’s Cost of Poor Quality at Lableaders.com). Soft failures result in delayed productivity and careless work, and they can lead to billing errors, excess supplies and unnecessary overtime.
In addition to CoPQ, laboratories must also acknowledge external failure costs, notably setbacks associated with defects after results have been released. She advises that lab managers must address them quickly to prevent reputational harm and avoid putting patients in danger. If overlooked, minor matters can become serious and expensive problems.
Pursue open and proactive communication
Culture is also key to optimising internal performance. To achieve a “culture of quality,” Dawson espouses a management framework called Just Culture, which encourages employees to voluntarily report failures in a blame-free environment (see Cultivating a Culture of Quality on Lableaders.com).

To ensure that employees communicate openly and proactively, Dawson suggests appointing a central lab leader to a senior management position. This person must have technician experience, knowledge of all ISO 15189 regulatory requirements, and exceptional communication skills. This person will work directly with key stakeholders to ensure that failure reports are used to inform performance improvements.
It is also crucial that labs look beyond internal processes and engage with other healthcare stakeholders, including physicians, executive management, and even shareholders. Labs managers should consider seeking their input when setting policies, allocating budgets and preparing financials.
Labs should also make efforts to articulate the value of their data, according to David Chou, Chief Information and Chief Digital Officer of Children’s Mercy Hospital in Kansas City, MO (see Leveraging Lab Data to Connect with the C-suite). If lab data indicates a rise in flu incidence and severity, for example, medical staff and hospital administrators should be informed so that they can prepare accordingly.
Peter Gershkovich, Director of Pathology Informatics at Yale University Medical School, agrees that labs need to communicate more with their key external stakeholders. “It’s very rare that you have a powerful lab with a presence in the C-suite,” he notes. If lab leaders can succeed in changing this, they will help realise improvements in patient care and cost savings across the organisation.
Maintaining balance in a changing world
To complicate matters even further, the global lab market is changing quickly. Many labs now face pressure to reduce staff, replace manual procedures with robots, increase testing menus and move towards precision medicine. At the same time, we must keep our staff happy, grow our services and continue providing value to both patients and physicians.
Below are some tips to help maintain balance in an era of rapid change:
- Prepare for reduced demand of classic tests such as immunochemistry, haemostasis, haematology, microbiology and self-monitoring blood glucose tests. Train your staff and get ready to do more point-of-care testing, molecular diagnostics and tissue diagnostics.
- Consider business management systems to achieve gains in productivity, efficiency, cost-effectiveness and speed. These can be especially useful in public and non-profit organisations.
- Expect consolidation in laboratories. As point-of-care testing increases, specialty tests will increasingly go through core labs to contain costs and streamline operations. If you build a great reputation doing the types of tests that people need the most—and will continue to need in the future—you can please your key customers.
- Consider automated equipment for sorting lab orders, running tests and n transporting, storing and retrieving samples. Negotiate with the vendor for the lowest possible price on these machines and train your lab staff to offer valuable additional services, such as screening, consulting, quality control and quality improvement.
These strategies will help you stay ahead of the game and adapt to the newest standards while maintaining a steady balance in quality, speed and cost.
This article is based on two presentations by Navapun Charuruks, MD, FRCPath (Thailand): (a) Cost Management for Labs at Roche Efficiency Days (RED) 2017 in Taipei, Taiwan; and (b) Challenges of Lab Services at Roche Scientific Days 2018: Empowering Lab Leadership to the Next Level in Dusit Thani Hua Hin, Thailand.