As Director of the Point-of-Care Testing Center for Teaching and Research (POCT•CTR) and Professor Emeritus of Pathology and Laboratory Medicine at the University of California Davis School of Medicine, Dr Gerald Kost is one of the world’s leading experts in point-of-care testing (POCT). He is also a Fulbright Scholar 2020-21 in ASEAN. In this Q&A, he shares insights for evaluating new POCT for COVID-19 management, implementing these tests in clinical practice, and developing policies to fuel diagnostic innovation and help prevent future outbreaks of highly infectious diseases.
What are some key considerations to effectively deploying POCT in the current pandemic?
An important consideration for evaluating a new COVID-19 test is the elimination of false negative test results. To do that, tests need to have high sensitivity, defined by the ratio of true positive results (TP) to the sum of true positive and false negative (FN) results [TP/(TP + FN)], whether the test is performed at the point of care or elsewhere.
Mathematical models of test performance are extremely important for setting testing strategy. The chart below looks at three different “tiers” of testing quality, showing the false positive to the true positive ratio (FP/TP), predictive value (PPV), negative predictive value (NPV), and the false omission rate (RFO) as a function of prevalence (from reference 1).
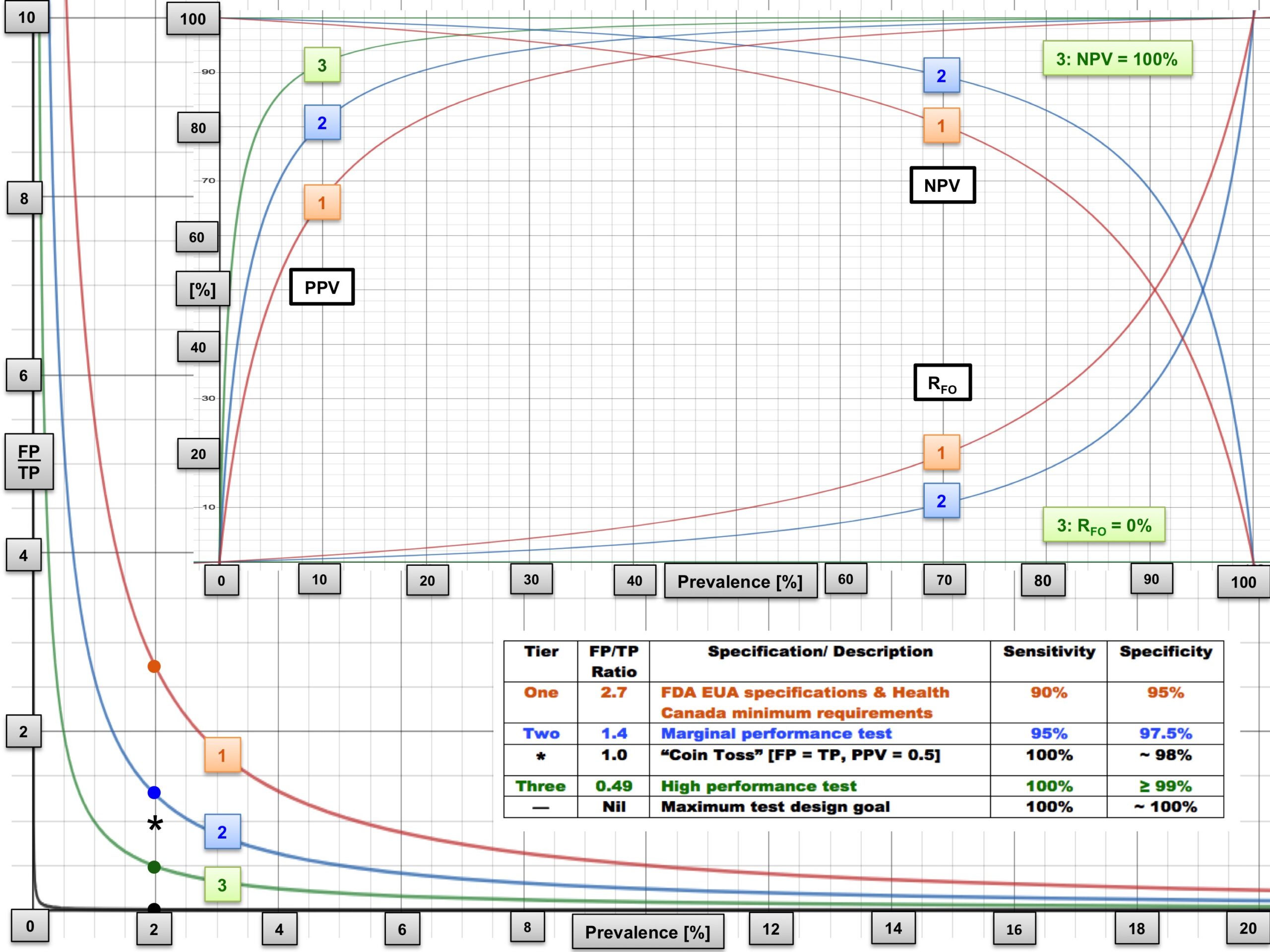
At a low prevalence of 2-5%, Tier 3 tests with very high sensitivity (100%) and specificity (≥99%) are needed. Most communities are experiencing low prevalence. In ERs, clinicians are seeing more infected patients, so the pre-test probability of COVID-19 increases, and as a result, the effective prevalence increases as well. Hence, prevalence depends on the setting.
What are some promising tests that authorities can consider for COVID-19 POCT?
Any test with objective confirmation of high performance in Tier 3 is very promising for full implementation in management of the current pandemic, as well as new outbreaks. This includes molecular diagnostic tests for primary detection of SARS-CoV-2 or antibody tests for assessment of the immune response following infection or immunisation.
Tests authorised by the Food and Drug Administration in the USA under Emergency Use Authorization rules can be found under reference 10. A word of caution is that many tests on the market do not perform well. POCT should not be an excuse for inaccuracy. A healthcare practitioner should evaluate the performance of POCT before implementing it for COVID-19.
How can clinical lab professionals contribute to POC deployment for COVID-19 management?
The dozen recommendations listed below show how laboratory professionals can deploy POCT to control the pandemic, help acutely infected patients, and expedite decision-making, therapy and recovery.
- Collecting specimens, screening and evaluating people safely in drive-up/in/throughs, walk-bys and pop-ups to prevent the spread
- Assuring high test performance with excellent positive predictive value [TP/(TP + FP)] and negative predictive value [TN/(TN + FN)]
- Relieving hospital infrastructure by limiting emergency room burden, unnecessary hospitalisation and readmission of low-risk patients
- Differentiating common Influenza A/B from COVID-19
- Discovering stealth COVID-19 transmission through widespread access to testing and self-testing
- Accelerating molecular diagnosis, triage, isolation and decision making for patients infected with SARS-CoV-2
- Assisting public health tracing of contacts of infected persons
- Monitoring “happy hypoxemia” (pulmonary compromise) using fingertip pulse oximetry (oxygen saturation monitoring)
- Diagnosing bloodstream pathogens, determining antimicrobial resistance and speeding targeted therapy for co-infections and sepsis
- Staging patients with pulmonary infections, and those critically ill with ARDS
- Measuring arterial blood gases and with inspired FiO2, determining the severity of ARDS using the P/F ratio (PaO2/FiO2): >200, mild; 100-200, moderate; and <100, severe
- Assessing viral loads during pharmacological treatment, IgG and IgM immunity during remission and antibody titers following vaccination
How can governments and policymakers support the use of POCT for COVID-19?
Governments and policymakers should fund research and development of POCT to detect disease, assess the immune response and develop strategies to couple diagnostics with therapeutic regimens. They should offer funding mechanisms and promote business models to help start-ups to invent new POCT for SARS-CoV-2. They should create national guidelines for testing and provide free access to testing for the general public. Importantly, they should launch public health campaigns to ensure the general public understands the purpose of POCT for disease surveillance, contact tracing and management.
What can we learn from past pandemics to inform the role of POC strategies in the COVID-19 response?
The Ebola epidemic in West Africa taught us crucial lessons in preparing for the COVID-19 crisis, but advice, strategies and technological development—especially in POCT—were mostly ignored. We must take action so this does not happen again.
For additional information, please refer to Global Point of Care—Strategies for Disasters, Emergencies and Public Health Resiliency, as well as references 7 & 8 below. Dr Mark Shephard’s A Practical Guide to Global Point-of-Care Testing also provides valuable material.
What can education institutions do to support training in POCT technologies and practices?
Schools of public health must modernise curricula to include training in POCT. The COVID-19 pandemic has shown unequivocally that POCT strategies are needed for detection of infection, contact tracing and documentation of immune response when people want to return to work. As POCT has not been emphasised in public health education, we are inadequately prepared in implementing POCT in the midst of the largest public health crisis of the century. For more discussion of POCT curricula and accreditation for public health, please see the references 5 & 6 below.
How can POCT systems be used to anticipate the next pandemic?
Nations are not ready for the next pandemic. The world has changed, and the POC profession must change with it. One way to implement change is through “point-of-careology,” a novel and also common sense concept for the future. Developed in China by a team led by Professor Xiguang Liu in Wuhan, point-of-careology is an emerging medical discipline that focuses on the role of POCT to quickly produce test results, accelerate therapeutic decision-making and reduce the economic burden of healthcare. Rapid, accurate and safe POCT would allow nations to contain the next infectious disease outbreak early before it spreads worldwide.
References
[1] Kost GJ. Designing and interpreting COVID-19 diagnostics: Mathematics, visual logistics, and low prevalence. Archives of Pathology and Laboratory Medicine. 2020. [open access]
[2] Kost GJ. Geospatial spread of antimicrobial resistance, bacterial and fungal threats to COVID-19 survival, and point-of-care solutions. Archives of Pathology and Laboratory Medicine. 2020. [open access]
[3] Kost GJ. Geospatial hotspots need point-of-care strategies to help stop highly infectious outbreaks: Ebola and Coronavirus-19. Archives of Pathology and Laboratory Medicine. 2020. [open access]
[4] Liu X, Zhu X, Kost GJ et al. The creation of point-of-careology. Point of Care. 2019;18(3):77-84. [open access]
[5] Kost GJ. Geospatial science and point-of-care testing: Creating solutions for population access, emergencies, outbreaks, and disasters. Frontiers in Public Health. 2019;7:329. [open access]
[6] Kost GJ, et al. POCT curriculum and accreditation for public health: Enabling preparedness, response, and higher standards of care at points of need. Frontiers in Public Health. 2019;6:385. [open access]
[7] Kost GJ. Molecular and point-of-care diagnostics for Ebola and new threats: National POCT policy and guidelines will stop epidemics. Expert Review of Molecular Diagnostics. 2018;18(7):657-673.
[8] Kost GJ et al. Molecular detection and point-of-care testing in Ebola virus disease and other threats: a new global public health framework to stop outbreaks. Expert Review of Molecular Diagnostics. 2015;15(10):1245-1259.
[9] Kost GJ, Curtis CM, Eds. Global Point of Care — Strategies for Disasters, Emergencies, and Public Health Resiliency. Washington DC: AACC Press-Elsevier, 2015. [contributed book, 701 pp.]
[10] Food and Drug Administration, United States. In vitro diagnostics EUAs. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas
Acknowledgements
This work was supported by the Point-of-Care Testing Center for Teaching and Research (POCT•CTR) and by Dr Kost, its Director. Figures and tables are provided courtesy and permission of Knowledge Optimization, Davis, California.





