The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) defines sepsis as a ‘life-threatening organ dysfunction caused by a dysregulated host response to infection’.
Septic shock is defined as ‘a subset of sepsis in which the underlying circulatory, cellular and metabolic abnormalities are associated with a greater risk of mortality than sepsis alone’. Sepsis is a clinical diagnosis, as there is no single diagnostic test. Both physiological and laboratory abnormalities may be present. Sepsis-3 eliminated the term ‘severe sepsis’, instead defining and stratifying ‘septic shock’ and ‘sepsis’ with the goal of facilitating the earlier recognition and more timely management of patients with sepsis. Sepsis presents as a spectrum of severity. Although there may be uncertainty about the diagnosis, the potential for rapid and serious deterioration in any patient with sepsis necessitates intervention whenever sepsis is a possibility [1].
The Global Burden of Disease study used data from 109 million death records and 8.6 million hospital records in 195 countries and territories to estimate the burden of sepsis around the world. It found there were 49 million sepsis cases and 11 million deaths in 2017 – double previous estimates – with as many as one in five deaths worldwide related to sepsis. It was concerning that so many lives were being lost to a condition that is largely preventable. Previous estimates of the burden of sepsis in Australia were mainly those treated in intensive care units, which put the number of cases at 18,000 and deaths at 5,000. But the Global Burden of Disease study estimate now gives a more accurate picture of the size of the problem by including sepsis occurring outside of the hospital, putting the number of Australian cases at 55,000 and deaths at 8,700.
Sepsis is a time-critical medical emergency that arises when the body’s response to an infection damages its own tissues and organs. It can lead to shock, failure of multiple organs, and death if not recognised early and not treated promptly. Sepsis affects people of all ages and patients across a broad range of clinical specialties but particularly the very young, the very old and Aboriginal and Torres Strait Islander peoples. However, awareness is low with a 2016 survey finding 60 percent of Australians had not heard of sepsis and only 14 percent could name one of its symptoms.There is a need for coordinated national approach that addresses pre-hospital and in-hospital recognition and treatment, to address the significant death and disability caused by sepsis [2].
Role of Procalcitonin in management of sepsis
Clinical management of critically ill patients with severe infection and sepsis can be improved by shortening the time to diagnostic and treatment decision (i.e. differentiation between bacterial vs. viral vs. fungal infection and vs. noninfectious etiologies). Furthermore, site-of-care decisions can be improved (e.g., early discharge or escalation of care) by an early risk stratification and the provision of prognostic information. Repeatedly measured biomarkers also help monitoring patients for tailoring therapy to individual needs of patients (antibiotic stewardship). In this context, the use of the host-response and blood infection marker procalcitonin (PCT) has gained much attention and has already been approved for guidance of antimicrobial therapies in patients with respiratory infection and sepsis. PCT is a precursor hormone of calcitonin that is not detectable in healthy individuals. However, the production of PCT is upregulated in response to bacterial infections and can decrease rapidly during recovery. Thus, PCT provides important additional information, which are able to supplement clinical and diagnostic parameters. This in turn, has not only a high impact on decisions regarding treatment of patients with suspected infections or sepsis, but can also influence the duration of antibiotic treatment courses. The use of PCT is evolving in the management of sepsis and several interventional studies and systematic reviews have analysed and summarised the effects of PCT guided strategies on antibiotic use and health outcomes. However, there is no universal consensus on the optimal use of PCT in the setting of sepsis.
In healthy individuals, serum PCT is not detectable, since the protein is not released into the blood in absence of systemic inflammation. In case of a sepsis caused by bacterial infections, however, PCT synthesis is induced in practically all tissues and therefore detectable in the blood. PCT synthesis is triggered by bacterial toxins, such as endotoxin and cytokines e.g., interleukin (IL)-1beta, interleukin-6 and tumor necrosis factor (TNF)-alpha. Due to cytokines released during viral infections that inhibit the production of TNF-alpha, PCT synthesis is not induced in most viral infections. Moreover, PCT has a wide biological range, a short time to induction after bacterial stimulation and a long half-life. Thus, PCT has good discriminatory properties for the differentiation between bacterial and viral inflammations with rapidly available results. PCT per se cannot isolate or detect specific pathogens, but the level of PCT may be useful to estimate the probability of a severe bacterial infection.
Despite decades of research efforts, there is still no sepsis specific treatment option available. Crucial for a successful treatment and positive outcomes, is an early diagnosis and differentiation from non-infectious causes, in order to rapidly start with antimicrobial therapy and fluid resuscitation. However, due to the fact that clinical signs for a definite or suspected sepsis can be heterogeneous and often ambiguous, its diagnosis and treatment remains challenging. To date, no gold standard exists for the detection of sepsis caused by bloodstream infections. The use of conventional diagnostic approaches such as blood cultures and inflammatory blood markers C-reactive protein (CRP), white blood count (WBC)] in patients with a clinical suspected infection or sepsis is restricted by some limitations. The use of blood cultures for the identification of pathogens, can provide information about type of microorganism and susceptibility towards antibiotic therapy. However, only a small part of the analysed cultures results positive and in around 40–90% of patients with an assumed systemic infection, the results are negative blood culture with no growing pathogens. Moreover, the long time to results limits initial treatment decision making and contamination leads to suboptimal specificity of the obtained results. In order to improve diagnostic work-up, additional tests are appropriate, which are able to facilitate an early and reliable diagnosis [3].
IL-6 and its role in neonatal sepsis
Neonatal sepsis accounts for 0.97% of all disability-adjusted life years worldwide, demonstrating higher percentages compared to colon and rectum cancer, asthma or breast cancer. Neonatal sepsis has a high mortality of 11–19%2 and is associated with brain injury as well as poor neurodevelopmental and growth outcomes in early childhood. These adverse outcomes call for early detection and rapid intervention. Since sepsis is a systemic inflammatory response to infection, isolation of bacteria from blood is considered the gold standard. Due to the low obtainable blood volume in very low-birthweight infants (VLBW infants, birth weight < 1500 gm) of around 0.5–1 ml, the high percentage of low-level bacteraemia and the long duration to a positive blood culture, several parameters have been tested for their accuracy in diagnosing neonatal sepsis including procalcitonin, lipopolysaccharide-binding protein, presepsin and interleukin-6.
IL-6 is a multifunctional cytokine participating in immune response, haemopoiesis and acute-phase reactions. In response to infection, IL-6 enhances the production of IgM, IgG and IgA as well as the proliferation of helper T-cells and thereby plays a crucial role in host defence mechanisms. After exposure to bacterial endotoxins, IL-6 concentrations rise before acute-phase reactants including C-reactive protein (CRP). IL-6 can be determined in cord blood or serum yielding different diagnostic accuracy. Cord blood IL-6 has a sensitivity of 87–100% for early-onset sepsis. Serum IL-6 has a sensitivity of 75–85% and a specificity of 72.8–88% for the diagnosis of early-onset sepsis and a sensitivity of 80–93.8% and a specificity of 80–96% for late-onset sepsis, thereby outperforming CRP. Serum IL-6 concentrations quickly fall to undetectable values during antibiotic treatment. The high sensitivity and negative predictive value of IL-6 make it a suitable candidate for diagnosing neonatal sepsis in combination with the high specific CRP.
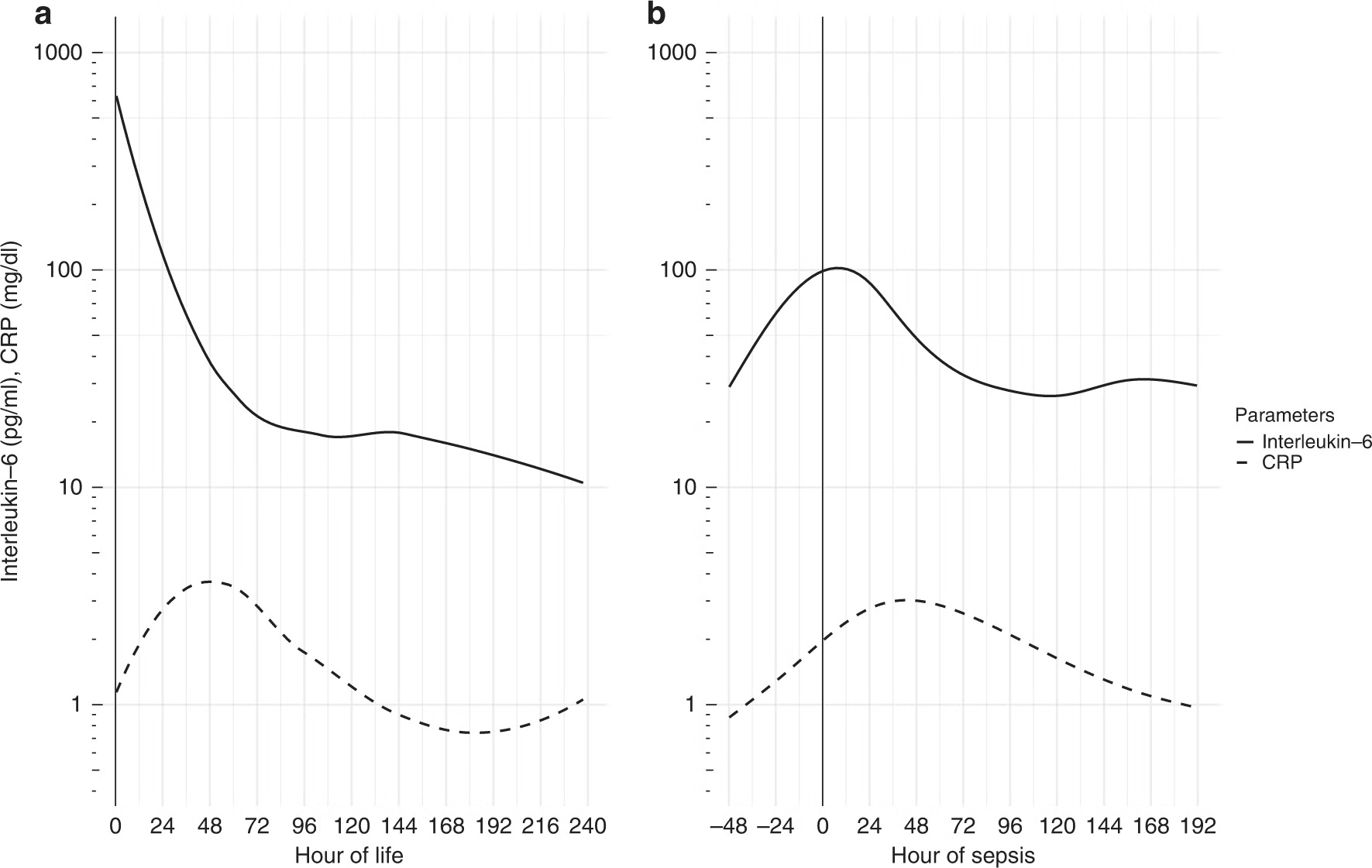
Fig1: Interleukin-6 and C-reactive protein and their development over time in sepsis. Serum interleukin-6 and C-reactive protein (CRP) in culture-confirmed sepsis on day of life 1 (a) and after day of life 7 (b).
As shown in Fig. 1, serum IL-6 starts highly elevated in cases of culture-confirmed sepsis on DOL 1( Day of Life 1), whereas CRP takes 48 h to reach its peak. Based on the high accuracy of serum IL-6, we conclude that IL-6 determined on DOL 1 is very helpful in the diagnosis of congenital sepsis. As also shown in Fig. 1, serum IL-6 increases 24–48 h before CRP in episodes of culture-confirmed sepsis after DOL 7. This makes serum IL-6 the most accurate and first laboratory parameter to rise in cases of sepsis after the first week of life. Tzialla et al. reported cytokine levels to change rapidly, even before acute-phase reactants.
Based on the high accuracy and especially the very high negative predictive value of serum IL-6, we conclude that serum IL-6 is very helpful in the diagnosis of sepsis in neonates and preterm infants. Furthermore, IL-6 falls rapidly after initiation of effective antibiotic therapy, which allows fast monitoring of antibiotic therapy and timely escalation or de-escalation of treatment [4].
Conclusion
Sepsis is a life-threatening disease and is one of the most common causes of ICU inpatients. Early diagnosis and proper management are necessary to reduce the mortality of sepsis. However, the individual difference in the physiological response to infection is large, and the signs and symptoms of sepsis are non-specific, making early diagnosis difficult. Therefore, a number of potential biomarkers for the diagnosis of sepsis have been investigated. These molecules are mainly involved in the initial pathogenesis of the innate immune response to infection, and in many cases, they show prognostic value as well as diagnostic value. The prognostic markers of sepsis are often involved in the organ dysfunction caused by sepsis and the development of therapeutic agents for sepsis targeting these prognostic biomarkers is being attempted. In addition, recent advances in technology have led to the development of new types of biomarkers such as the microbiome and non-coding RNAs. The intestinal microbiome is known to play an important role in the development and maturation of the immune system and protection against pathogens. Therefore, gut dysbiosis is considered to be a powerful biomarker for the development and progression of sepsis. Non-coding RNAs including miRNAs and long non-coding RNAs regulate gene expression in various ways, but their function and mechanisms in the pathogenesis of sepsis are not completely understood.
Further evaluation, including the roles of these new biomarkers in the pathogenesis of sepsis and the development of an optimal normalisation strategy for the analysis of these new biomarkers will be needed for their future clinical use [5].
References:
[1] Sepsis Clinical Care Standard 2022
[2] Global Burden of Disease study
[3] Gregoriano, C. et al. (2020) ‘Role of procalcitonin use in the management of sepsis’, Journal of Thoracic Disease, 12(S1). doi:10.21037/jtd.2019.11.63.
[4] Küng, E. et al. (2022) ‘Cut-off values of serum interleukin-6 for culture-confirmed sepsis in neonates’, Pediatric Research, 93(7), pp. 1969–1974. doi:10.1038/s41390-022-02329-9.
[5] Kim, M.-H. and Choi, J.-H. (2020) ‘An update on sepsis biomarkers’, Infection & Chemotherapy, 52(1), p. 1. doi:10.3947/ic.2020.52.1.1.
This article originally appeared on Roche Diagnostics Australia.








