Over 90% of clinical labs in the Asia Pacific region have a laboratory information system (LIS) to manage their data and workflows, but less than half use middleware to integrate disparate systems. Yet middleware adoption is growing quickly, according to the latest results from the Asia Pacific Laboratory Benchmarking Survey, an annual survey by Roche Diagnostics that measures the operational effectiveness of clinical labs across the region.
Rising Middleware Adoption
In the most recent survey, 46% of all the labs in the Asia Pacific region said they were using or planning to use middleware—up from only 29% in 2017. As shown in the charts below, penetration of middleware systems is slightly greater in developed countries, but a slightly larger share of labs in developing markets are planning to install middleware systems in the coming years.
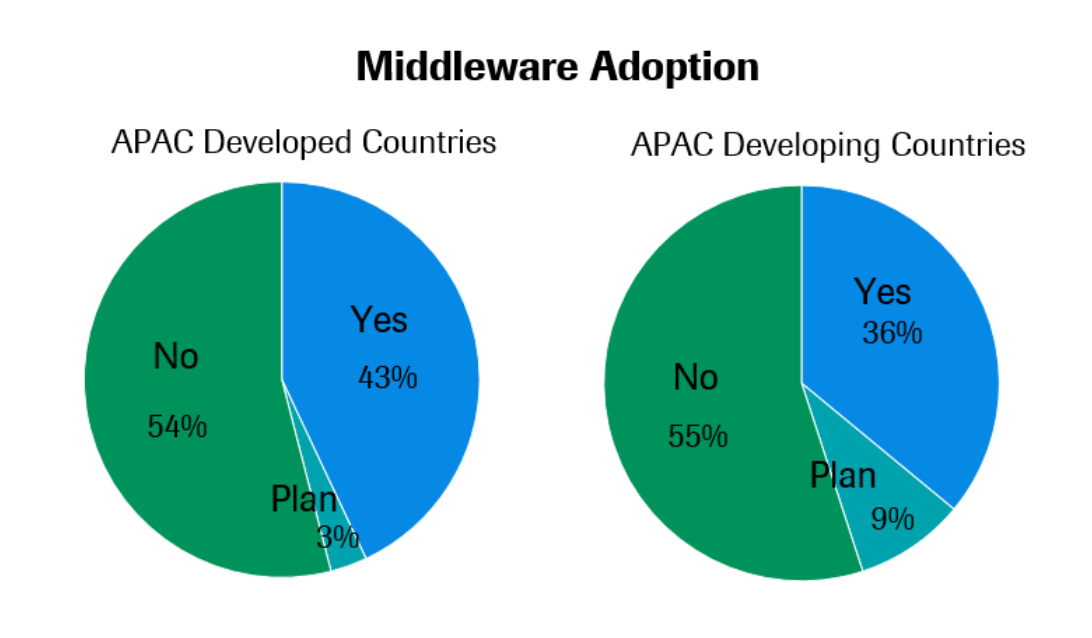
Despite growing adoption in Asia Pacific, middleware is still more widely used in the United States and Europe, according to Associate Professor Sunil Sethi, President of the Asia Pacific Federation of Clinical Biochemistry and Laboratory Medicine (APFCB) and Group Director of Laboratory Medicine at the National University Health System (NUHS) in Singapore.
“We still don’t see [middleware] very much in our part of the world,” said Sethi at the recent Roche Efficiency Day event in Nagoya, Japan in December 2019 (see Laboratory Automation, Process Control and Seamless Informatics with LIS/EMR/NEHR Integration for further details about how his organization is using middleware to connect disparate systems and streamline important tasks).
LIS and Middleware Uses
Labs in the Asia Pacific region use LIS and middleware systems for a wide range of purposes. The five most common uses include turnaround time monitoring (75.9%), critical results reporting (73.2%), generation of statistics reports (71.4%), data export (70.1%) and QC reports/statistics (69.3%).
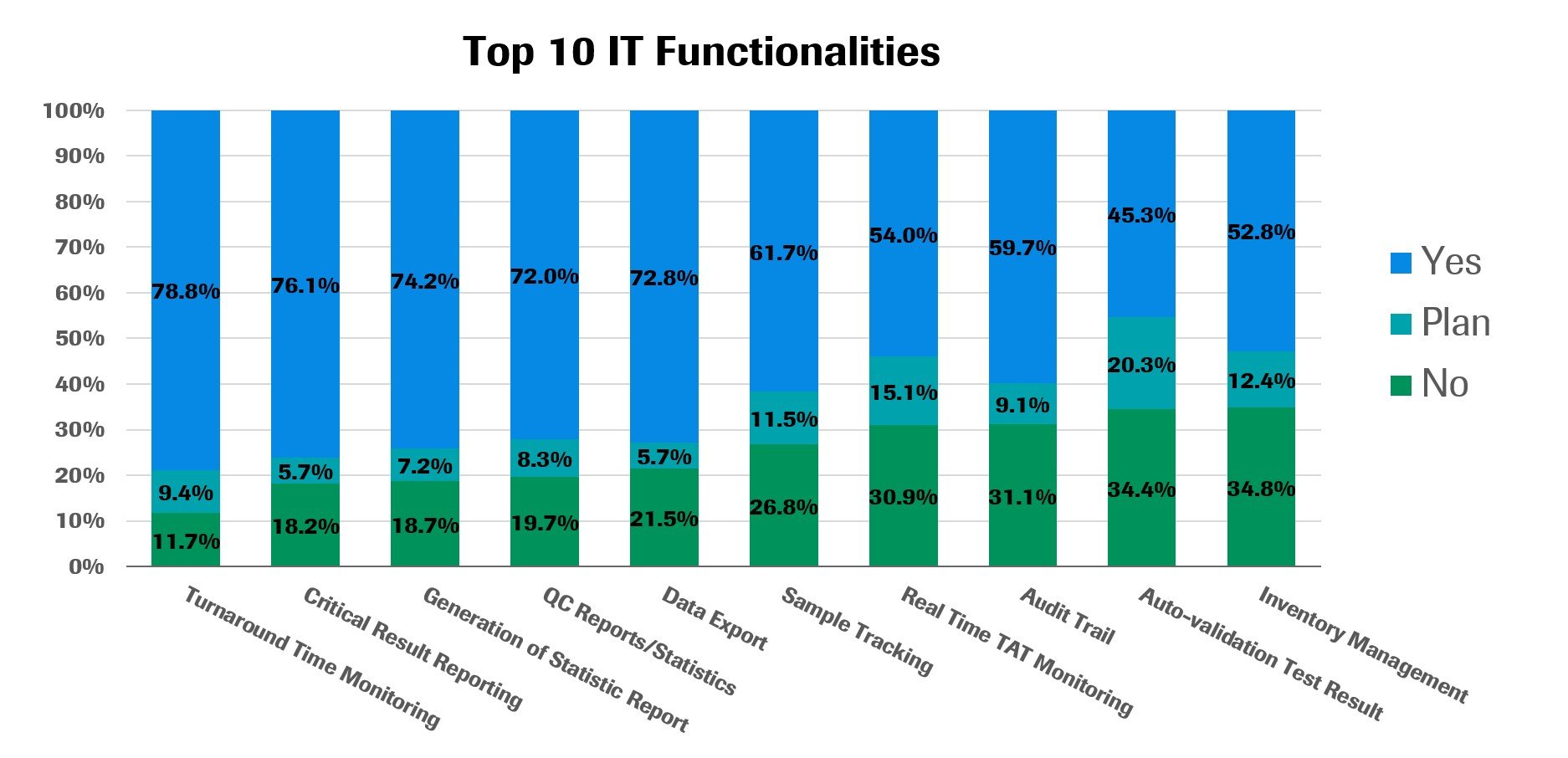
Real-time TAT monitoring (52.0%), inventory management (50.9%) and auto-validation (43.6%) are less commonly used, but interest in these technologies appears strong, as an above-average share of respondents indicate they plan to adopt them in the coming years. Less common uses of IT systems include patient result management (46.4%), accounting/billing for tests (44.6%), serum indices interpretation (39.2%), KPI dashboards (25.4%), smartphone notifications (17.4%), and AI-assisted clinical decision making (9.9%).
When reviewing these results, one thing to note is that large labs (defined as those running more than 1000 samples/day) are more likely to have IT systems in place, whereas smaller labs are less likely to use them.
For more detailed data and to keep on top of these trends, please register for an account on Lab Insights and subscribe to our Roche Diagnostics Asia Pacific LinkedIn channel for updates. And if you have any specific questions about what these trends mean for your lab, contact Wesley Wong ([email protected]) for more information.








