A large majority of clinical labs in the Asia Pacific region maintain turnaround time (TAT) targets for cardiac biomarkers, but some countries and laboratory market segments have more ambitious targets than others, according to the latest results from the Asia Pacific Laboratory Benchmarking Survey, an annual survey by Roche Diagnostics that measures the operational effectiveness of clinical labs across the region.
The Value of TAT Targets
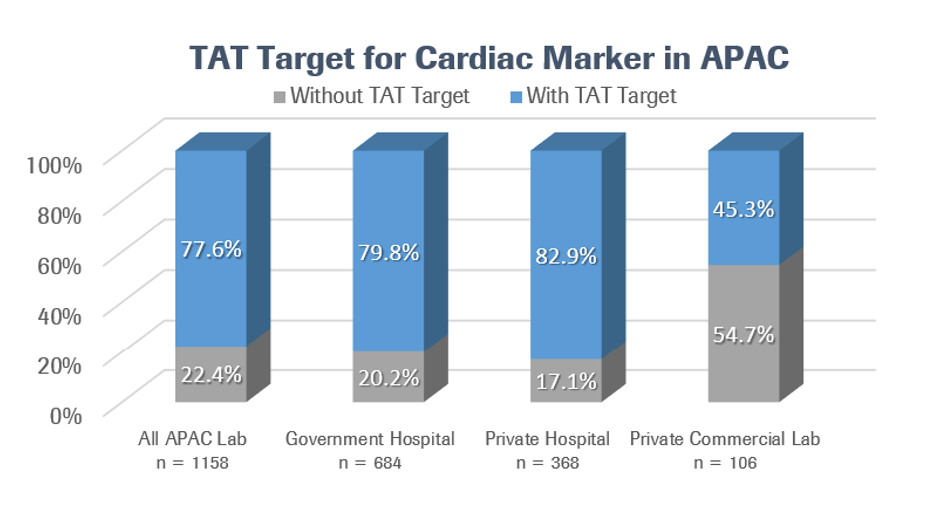
Among the 1158 clinical labs in the region that participated in the most recent benchmarking survey, 77.6% had a TAT target for cardiac biomarkers. A much higher share of government and private hospital labs had TAT targets than private commercial labs, presumably because hospital labs are more likely to deal with time-sensitive results in emergency and acute care settings, which can sometimes have life-or-death consequences.
Even in non-emergency situations, faster turnaround times can have a positive impact on patient experience and outcomes. When patients do not have to wait long for results, physicians feel more comfortable offering cardiac biomarker tests in the clinic (see Transforming heart failure care at Huelva University Hospital for a case study on how a Spanish hospital used NT-proBNP testing in its primary care facilities to support heart failure management).
A Need for Speed
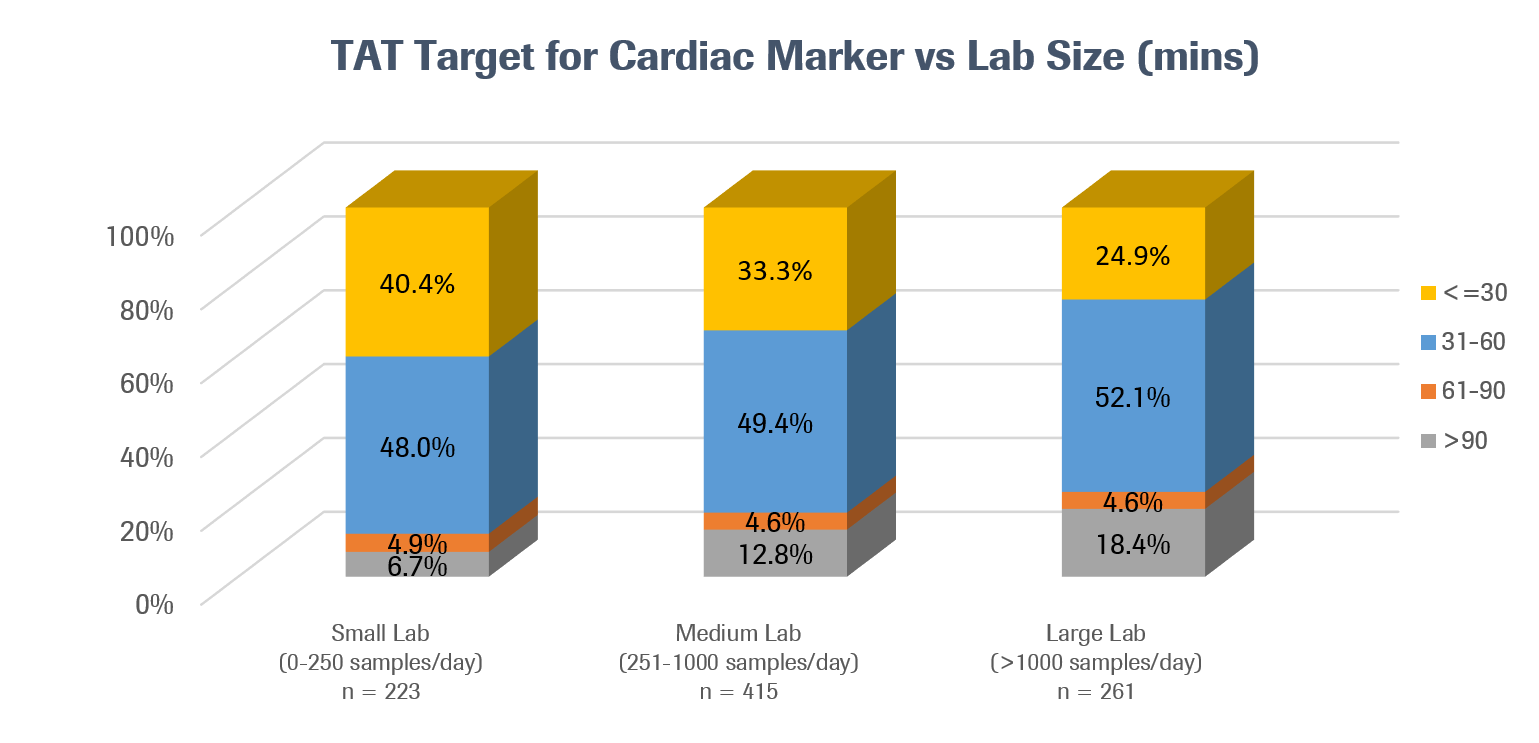
Among the 899 hospital and commercial labs across the region that had TAT targets for cardiac biomarkers, 32.6% of labs aimed to deliver results in less than 30 minutes. Small labs (defined as those that process less than 250 samples per day) were more likely to pursue this target than medium and large labs.
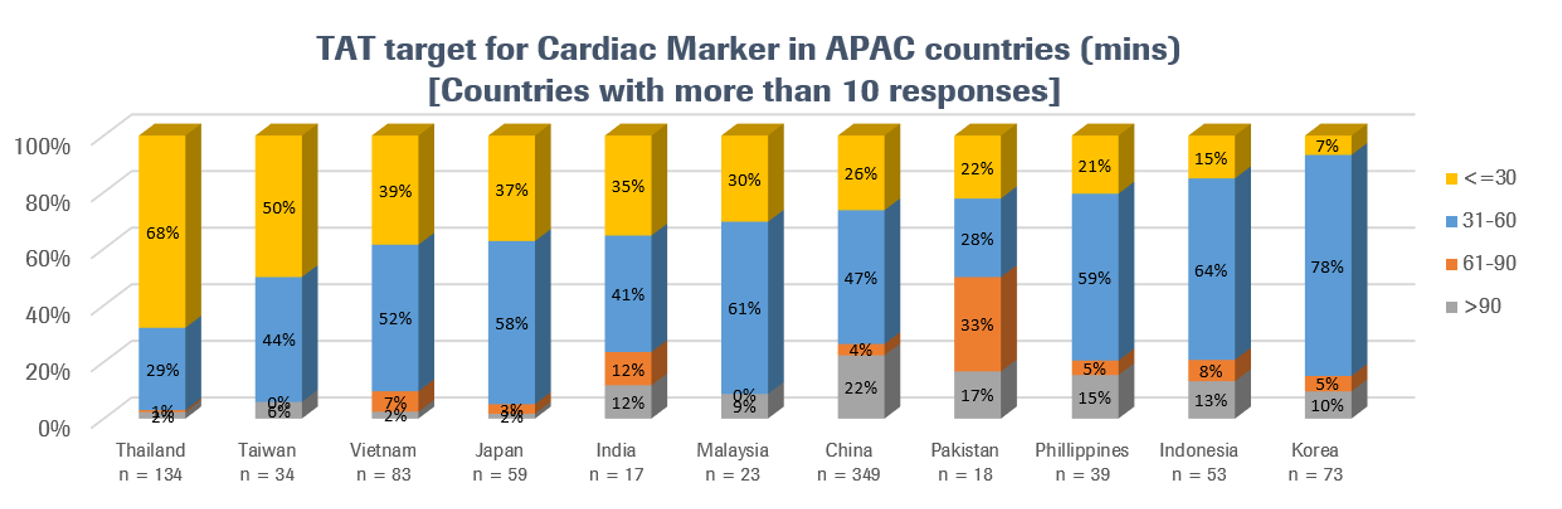
Most labs in most countries tend to have TAT targets of less than one hour, but some countries strive for faster performance than others. Labs in Thailand, for example, appear to place a particularly high priority on rapid TAT for cardiac biomarker results (see Eliminating diagnostic delay with point-of-care technology for acute coronary syndrome for a case study on how one university hospital lab in Thailand incorporated troponin T testing at the point of care to increase diagnostic speed and reduce patient mortality). Please note, however, that differences in the profile of respondents may impact these country comparisons, and that more data is needed to validate and explain these numbers.
Looking specifically at hospital labs, the survey results indicate that those with faster TAT tend to also make larger investments in some types of information technology, such as critical results reporting systems, auto-validation tools and nurse alert stations. These same labs are also more likely to invest in dedicated instruments and a dedicated lab for STAT samples.
Key Considerations and Disclosures
In interpreting this data, it is important to note that the data in this article refer to TAT targets only, not actual TAT performance. The data also does not specify between different cardiac biomarkers, and only offers a high-level indicator of clinical lab performance in this area. Depending on the context, TAT targets for key biomarkers like troponin or NT-proBNP may differ significantly between and within healthcare institutions.
In addition, most of the data was collected before the SARS-CoV-2 pandemic, and although some cardiac biomarkers may have a role in the management of COVID-19 patients, the current situation has undoubtedly impacted hospital and laboratory workflows (see COVID-19 in Wuhan: local cardiologist shares frontline experience for further discussion of the interplay between heart disease and mortality from COVID-19). As new data and case studies emerge, the Lab Insights team will continue to report on changes in diagnostic practices and workflows in cardiology.