To ensure quality and accuracy in their operations, many labs around the world follow standards set by the International Organisation for Standardisation (ISO). These standards include ISO/IEC/17025 for all types of testing and calibrating laboratories, and the ISO 15189 standards specifically for medical laboratories.
Meeting ISO standards can be a comprehensive, expensive and resource-intensive process. While large laboratories often have the resources to conform to these standards, smaller ones are sometimes discouraged by the costs and complexity of the process. In many cases, this has led to an “all or none” situation in many countries.
While ISO standards are valued internationally, mandatory minimal national quality standards can also play a role in ensuring quality. That’s why Thailand chose to implement a Thai Medical Technology (MT) Standard that sets a basic benchmark for quality.
The Thai Medical Technology (MT) Standard
Our concept for the Thai MT Standard was based on six key points:
- Simple to understand
- Low-cost to implement
- Feasible for labs at every level
- Compliant with the International Standards
- Compliant with the Thailand National Health Policy
- Every hospital will undergo Hospital Accreditation (HA) process
In developing these standards, the biggest challenge was syncing the international accreditation process with our own cultural requirements, particularly since the auditor was unable to provide guidance for our specific culture and country requirements. We want all our labs to be certified, but continuous quality improvement in lab services is the ultimate goal.
Thailand has 1,450 clinical laboratories. Two-thirds of those labs are in the government sector under the Ministry of Public Health and the rest are in university hospitals, regional medical science centres, military hospitals and the Bangkok Metropolitan Administration. We aimed to make the Thai MT Standard within reach for many of them.
Implementation, assessment, results
To implement our Thai MT Standard, we created an implementation roadmap that looked at strategic activities in four key areas: training, laboratory networks, internal quality audit and accreditation. Our laboratory assessment process included a one-day assessment with the number of auditors matching the size of the hospital or lab service.
We assessed the quality management system and technical and safety system, using a 100-clause checklist developed from ISO 15190 to measure quality. The cost of each assessment trip varied by the size of the lab, ranging from USD $345-645 per assessment. Labs that passed would receive a certification that is valid for three years.
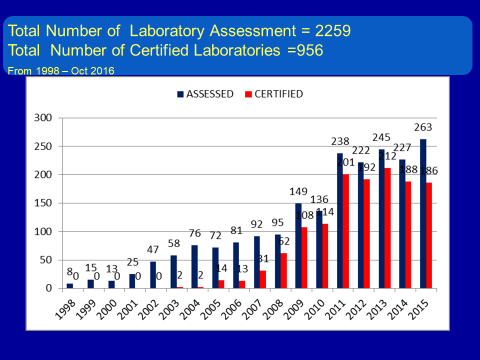
From 1998 to Oct 2016, the number of labs requesting an assessment has continually increased. In that period, there were 2,259 laboratories assessed, of which 956 were certified. Of the certified labs, 36% are to be continued for re-accreditation. All certified labs are listed in the Medical Technology Council directory online.
The Thai MT Standard and accreditation process helped spread awareness of quality. It fostered conceptual thinking about quality in both processes and services. It also led to improvements in facilities, safety protocols and managerial systems.
Participating labs also improved their workloads, purchasing and inventory controls, and laboratory information systems. They reduced turnaround time and increased quality assurance (IQC and EQA). Customer feedback also improved with greater satisfaction and reduced complaints.
This article is based on the presentation “Thailand Laboratory Benchmarking Survey 2014” at Roche Efficiency Days (RED) 2016, Beijing, China.








