The molecular pathology laboratory at Atrium Health is one of the largest full-service molecular pathology labs in the US and receives samples from the states of North and South Carolina. We run 750,000 tests per year and are very involved in clinical trial programs. In addition, the cytogenetics laboratory is one of the largest non-commercial laboratories in the US. The volume and requirements of such testing are demanding, and to improve workflow we implemented Lean Six Sigma (LSS) principles, which are designed to streamline work processes and reduce waste.
Given the wide range of tests we offer, we had many processes to evaluate. This includes tests for inherited diseases, infectious diseases, haematological and solid tumour oncology, chromosome analysis, fluorescence in situ-hybridisation analysis (FISH) and molecular cytogenetic analysis. Culturing and complicated analysis means that automation and staffing are the biggest challenges for our labs.

A few years ago, our facility experienced a large expansion in the number of tests we were completing, and the infrastructure of the lab was no longer able to handle the demand. We also had laboratories in various hospitals and on various floors – the cytogenetics laboratory was in the basement of the rehab building, the microbiology laboratory was in the main hospital, and the molecular pathology laboratory was in a third hospital.
In 2015, we decided to find additional space to bring all the laboratories together, designing an efficient layout based on Lean principles. Our goal was to improve workflow, increase productivity, cross-train staff and incorporate automation. The design strategy involved an efficient layout that would maximise our options for future reconfiguration as the need arises.
We enacted a Plan-Do-Study-Act (PDSA) cycle of change. Everyone who would later work in the newly designed building underwent Lean training in groups over a period of several months. This included not only laboratory staff, but also security, IT, and maintenance employees. Everyone was involved in the design process – it was teammate driven, and the lab technologists themselves designed the lab.
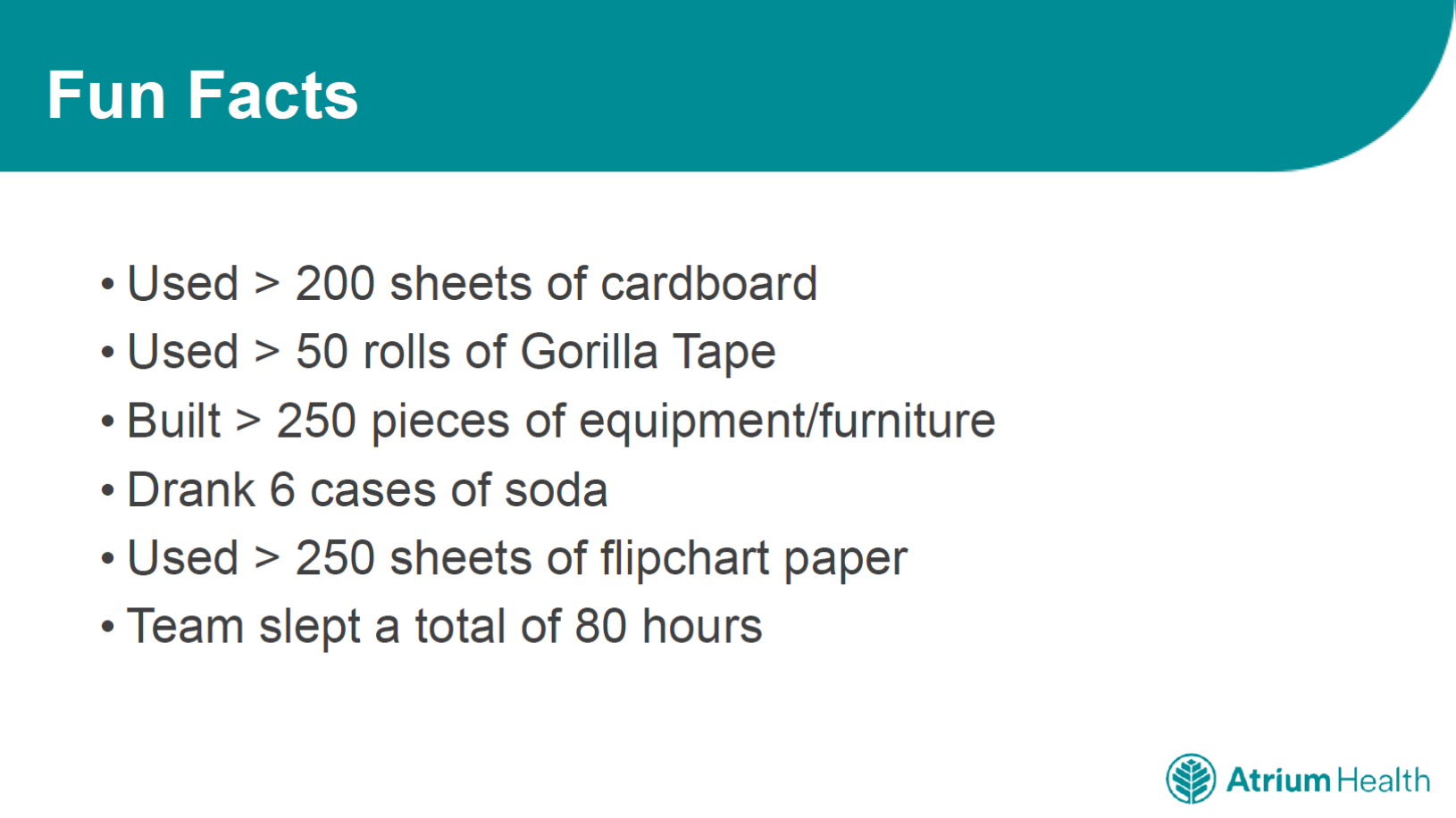
All of the teams even built life-size models of lab equipment from cardboard, placing every piece in the space to model how the workflow would work. A lot of time and energy went into the project. For example, just one team used more than 200 sheets of cardboard, built 250 pieces of furniture, and slept about 80 hours over a five-day period. The design process is hard work, but it can also be a fun and creative process that boosts team spirit.

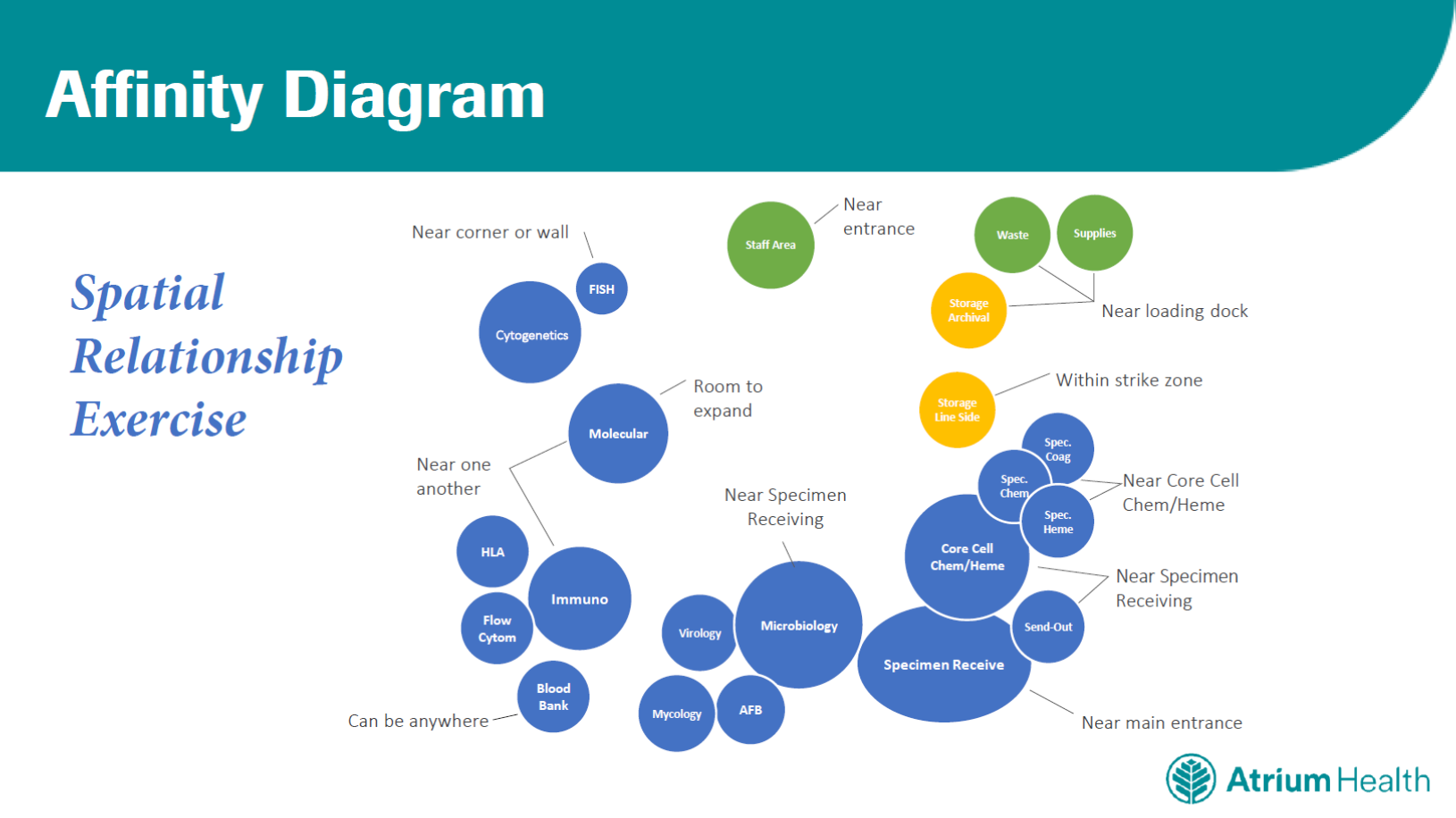
Most of the labs went through 9 to 12 design iterations, and a spatial relationship exercise was crucial in determining the most practical location and proximity of certain facilities. Specimens, equipment and supplies, for example, enter in the back of the building, near the loading dock. To reduce the handling distance for samples, the microbiology facility is close to the specimen management area. And to address the issue of stored supplies taking up too much lab space, we set up a “supermarket” area – essentially a large warehouse for supplies.
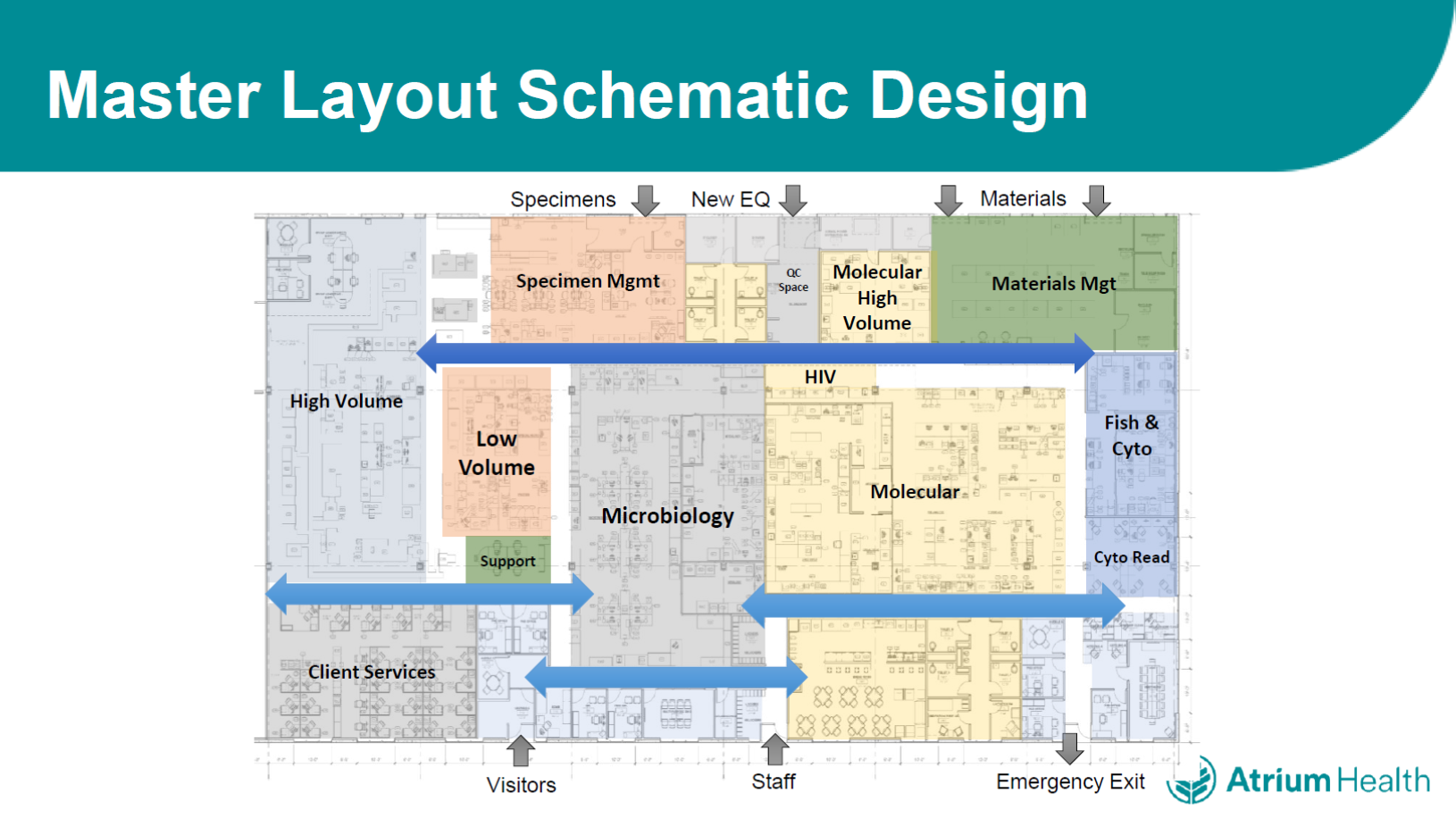
In February 2015, my cytogenetics laboratory was one of the first to move in to the new space, and we noticed significant improvements attributable to the new lab design, including a vastly more efficient supply chain and a smoother workflow. Travel distance for technicians within the laboratory dropped by 80-90%, and specimen travel distance was reduced by 86%.
The move also led to staffing improvements: supply storage in the supermarket freed technologists to focus purely on their technical work, which greatly increased turnaround time, morale and engagement. The ability to cross-train staff and to incorporate more automation into various labs, such as in the microbiology and molecular pathology labs, greatly reduced bottlenecks.

The change to a Lean lab environment constituted a paradigm shift for our lab’s employees. It enabled staff to work collaboratively and communicate more effectively, leading to time savings and improved testing flow. For any lab wanting to improve its efficiency, applying Lean principles can be a beneficial and practical solution.
This article is based on the presentation “Continuous process improvement in cytogenetics and molecular pathology” at the Roche Efficiency Days (RED) 2018 REDefining perspective in Guangzhou, China.








