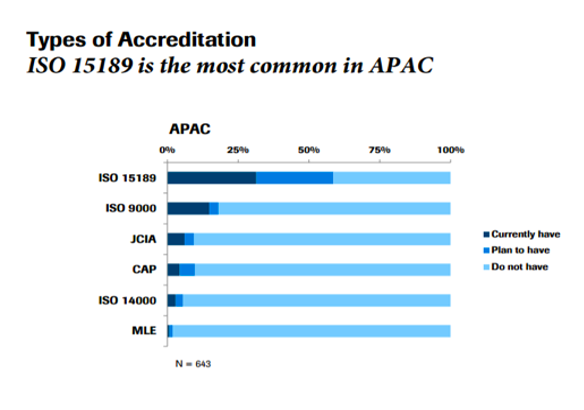
Laboratories in the Asia Pacific (APAC) region are increasingly seeking accreditation to build trust with physicians, patients and the many other stakeholders that rely on them to deliver accurate, timely results. While several types of accreditation are available for APAC medical labs, the one most commonly pursued is ISO 15189.
Currently, more than 25% of the labs in the region are ISO 15189 accredited—and that number is expected to double in the next few years, according to the 2014-5 results of the Asia Pacific Laboratory Benchmarking Survey, an annual survey by Roche Diagnostics that measures the operational effectiveness of clinical labs across the region.
If your laboratory elects to seek ISO 15189 accreditation, it must commit to rigorous internal quality control (IQC) and external quality assurance (EQA) programmes. Taken together, they let physicians and other healthcare professionals know that they can rely on your lab results to make safe decisions about the diagnosis and treatment of their patients.
Internal Quality Control (IQC) Standards
The purpose of IQC is to verify the calibration curve of an assay. According to ISO guidelines [1], “the laboratory should design quality control procedures that verify the attainment of the intended quality of results.” Adding further guidance, CLIA states [2] that “laboratories must test controls at prescribed intervals…at least two different concentrations should be used.” Further guidance on IQC practices can be sought from relevant professional societies.
It is also recommended that you run at least two levels for linear calibration curves and three levels for non-linear assays. If you find out-out-control values, record the value and check the procedure or instrument; do not simply run a new QC.
IQC is critical but also has some limitations. It only detects change in performance between current and “stable” operations. If the original determination of the target and range included systematic errors, it won’t be detected. This is why external quality assurance (EQA) is also important.
External Quality Assurance (EQA)
EQA is the process by which the results of your laboratory are compared with those of other laboratories performing the tests with the same methodology. EQA supplements IQC procedures by obtaining consensus values when true values are unknown. It also allows for investigation into performance factors (methods, staff) and acts as an educational stimulus to improvement in performance.
These programmes can be costly, but the costs of not practicing them—such as repeat testing, loss of credibility, and sub-optimal patient care—are often far higher.
Five “First Steps” to Quality
For medical laboratories in APAC, there is little doubt that accreditation is the way forward. And you can start down the path of improving quality—and increasing your confidence and pride in your work—by following these five steps right away.
- Work toward a standard of practice
- Perform IQC with every run
- Participate in an EQA
- Interpret against an appropriate decision limit
- Strive for ISO 15189 accreditation
This is not an easy path for laboratories with limited budgets and resources, but it is a necessity for laboratories who wish to succeed in an increasingly value-based, patient-centric healthcare market. After all, a well-managed laboratory is the one that understands the interdependence of cost, quality, and speed. Laboratories that prioritise these factors equally will be the ones best positioned to emerge as winners in the marketplace.
[1] ISO Guidelines for ISO 15189, Medical laboratories – Requirements for quality and competence
[2] CLIA Law & Regulations, Centre for Disease Control and Prevention
This article is based on the presentation “ISO 15189 Accreditation” at Roche Efficiency Days (RED) 2016 in Beijing, China.